Bischofite: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
No edit summary |
||
| (13 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
back to [[Chloride]] | |||
{{Infobox_Salt | {{Infobox_Salt | ||
|Footnote = <ref> http://webmineral.com/data/Bischofite.shtml viewed on 29/07/2010</ref><ref> http://www.mindat.org/min-681.html viewed on 29/07/2010</ref> | |Footnote = <ref> http://webmineral.com/data/Bischofite.shtml viewed on 29/07/2010</ref><ref> http://www.mindat.org/min-681.html viewed on 29/07/2010</ref> | ||
|photo = <!-- [[Image:"file name"|300px]]--> | |photo = <!-- [[Image:"file name"|300px]]--> | ||
|mineralogical_Name = Bischofite | |mineralogical_Name = Bischofite | ||
|chemical_Name = | |chemical_Name = Magnesium chloride hexahydrate | ||
|Trivial_Name = | |Trivial_Name = | ||
|chemical_Formula =MgCl<sub>2</sub>•6H<sub>2</sub>O | |chemical_Formula =MgCl<sub>2</sub>•6H<sub>2</sub>O | ||
|Hydratforms = | |Hydratforms = | ||
| | |Crystal_System =monoclinic | ||
|Deliqueszenzhumidity = | |Crystal_Structure = | ||
|Solubility = | |Deliqueszenzhumidity =33.1% | ||
|Density =1 | |Solubility =5.75 mol/kg | ||
|MolVolume =129 | |Density =1.57 g/cm<sup>3</sup> | ||
|Molweight =203 | |MolVolume =129.6 cm<sup>3</sup>/mol | ||
|Molweight =203.30 g/mol | |||
|Transparency =transparent to translucent | |Transparency =transparent to translucent | ||
|Cleavage =none | |Cleavage =none | ||
|Crystal_Habit = | |Crystal_Habit = | ||
|Twinning = | |Twinning = | ||
|Refractive_Indices =n<sub>x</sub> =1 | |Refractive_Indices =n<sub>x</sub> =1.495<br> n<sub>y</sub> = 1.509<br> n<sub>z</sub> = 1.528 | ||
|Birefringence =Δ = 0 | |Birefringence =Δ = 0.003 | ||
|optical_Orientation =positive | |optical_Orientation =positive | ||
|Pleochroism = | |Pleochroism = | ||
|Dispersion = | |Dispersion =79° | ||
|Phase_Transition = | |Phase_Transition = | ||
|chemBehavior = | |chemBehavior = | ||
|Comments = | |Comments =decomposes at 116-118°C<br>hygroscopic and deliquescent | ||
|Literature = <bib id="Broul.etal:1981"/> <bib id="Dana:1951"/> <bib id="Steiger.etal:2011a"/> | |||
}} | }} | ||
==Solubility== | |||
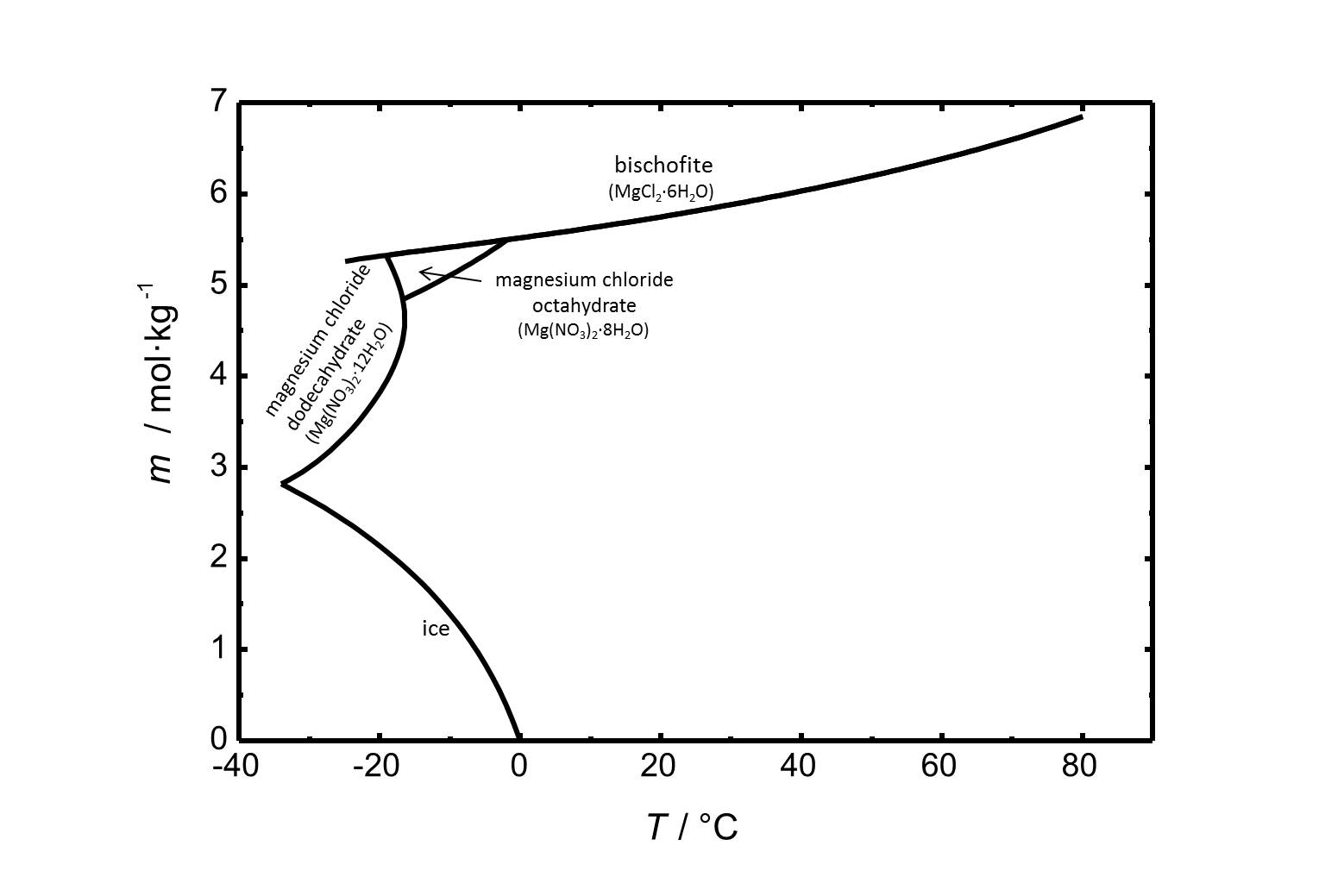
Bischofite has got a high solubility in water (5.75 mol/kg at 20 °C). The solubility of bischofite and other phases of the system MgCl<sub>2</sub>-H<sub>2</sub>O is shown in Fig. 1. <br> | |||
[[Image:MgCl2 S.jpg|thumb|left|800px|Figure 1: Solubility of magnesium chloride in water. The molality ''m'' [n(MgCl<sub>2</sub>)•kg(H<sub>2</sub>O)<sup>-1</sup>] is plotted versus the temperature.]] | |||
<br clear=all> | |||
==Hygroscopicity== | |||
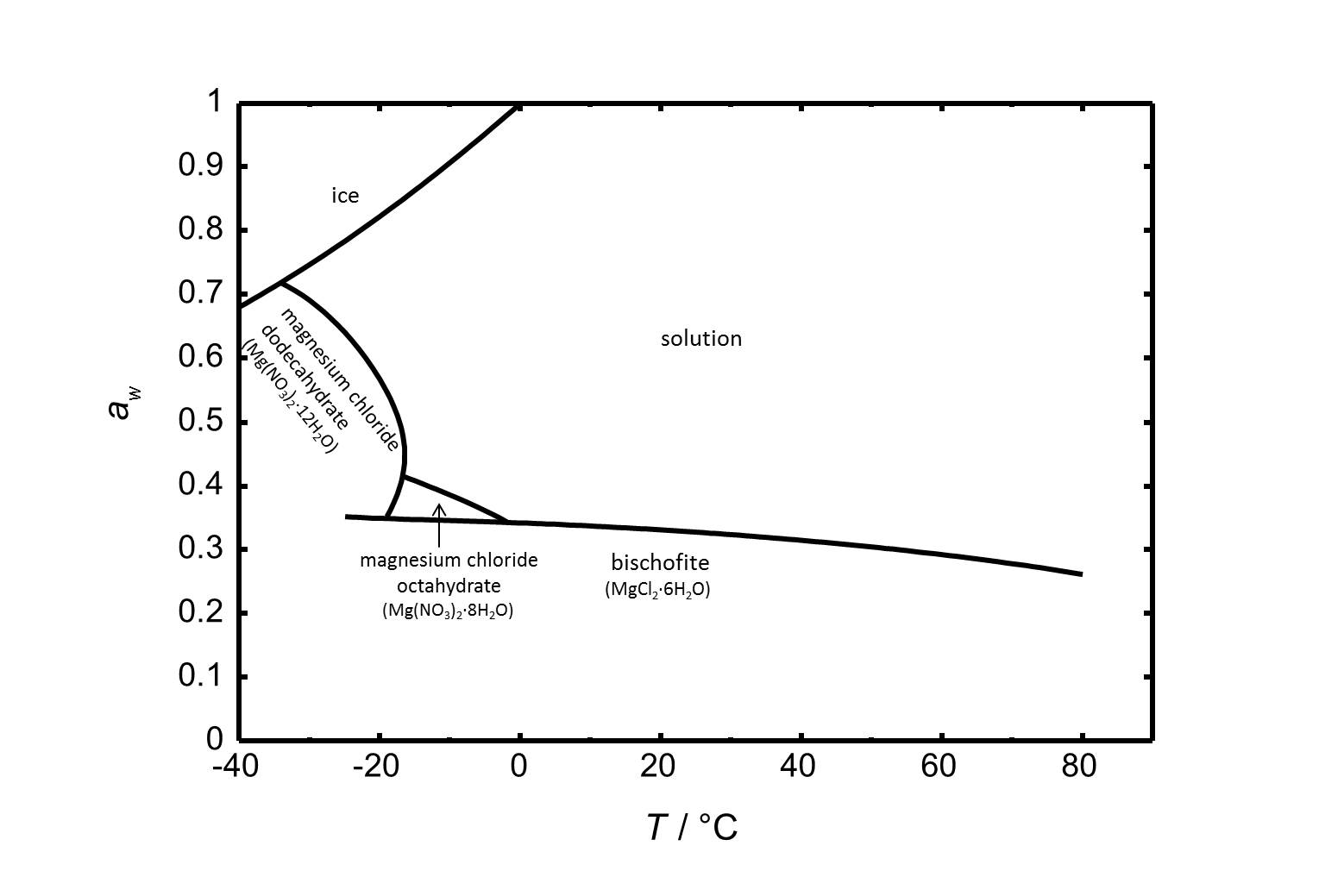
Figure 2 includes the phase diagram of the system MgCl<sub>2</sub>-H<sub>2</sub>O. Next to the hexahydrate bischofite there are two more stable phases within the regarded temperature range. The octa- and the dodecahydrate are relevant at lower temperatures. <br> | |||
[[Image:D MgCl2 e.jpg|thumb|left|800px|Figure 2: Phase diagram of the system MgCl<sub>2</sub>-H<sub>2</sub>O.The water activity ''a<sub>w</sub> is plotted versus the temperature.]] | |||
<br clear="all"> | |||
<br clear=all> | |||
{|border="2" cellspacing="0" cellpadding="4" width="52%" align="left" class="wikitable" | |||
|+''Table 1: Deliquescence humidities of bischofite at different temperatures <bib id="Steiger.etal:2014"/>'' | |||
|- | |||
|bgcolor = "#F0F0F0" align=center| 0°C | |||
|bgcolor = "#F0F0F0" align=center| 10°C | |||
|bgcolor = "#F0F0F0" align=center| 20°C | |||
|bgcolor = "#F0F0F0" align=center| 30°C | |||
|bgcolor = "#F0F0F0" align=center| 40°C | |||
|bgcolor = "#F0F0F0" align=center| 50°C | |||
|- | |||
|bgcolor = "#FFFFEO" align=center| 34.1%r.h. | |||
|bgcolor = "#FFFFEO" align=center| 33.7%r.h. | |||
|bgcolor = "#FFFFEO" align=center| 33.1%r.h. | |||
|bgcolor = "#FFFFEO" align=center| 32.4%r.h. | |||
|bgcolor = "#FFFFEO" align=center| 31.5%r.h. | |||
|bgcolor = "#FFFFEO" align=center| 30.5%r.h. | |||
|} | |||
<br clear=all> | |||
==Weblinks== | ==Weblinks== | ||
<references/> | <references/> | ||
[[Category:Bischofite]][[Category:Chloride]][[Category:Salt]][[Category:InProgress]] | == Literature== | ||
<biblist/> | |||
[[Category:Bischofite]][[Category:Chloride]][[Category:Salt]][[Category:InProgress]][[Category:List]] | |||
Latest revision as of 11:32, 3 May 2023
back to Chloride
| Bischofite[1][2] | |
| Mineralogical name | Bischofite |
| Chemical name | Magnesium chloride hexahydrate |
| Trivial name | |
| Chemical formula | MgCl2•6H2O |
| Other forms | |
| Crystal system | monoclinic |
| Crystal structure | |
| Deliquescence humidity 20°C | 33.1% |
| Solubility (g/l) at 20°C | 5.75 mol/kg |
| Density (g/cm³) | 1.57 g/cm3 |
| Molar volume | 129.6 cm3/mol |
| Molar weight | 203.30 g/mol |
| Transparency | transparent to translucent |
| Cleavage | none |
| Crystal habit | |
| Twinning | |
| Phase transition | |
| Chemical behavior | |
| Comments | decomposes at 116-118°C hygroscopic and deliquescent |
| Crystal Optics | |
| Refractive Indices | nx =1.495 ny = 1.509 nz = 1.528 |
| Birefringence | Δ = 0.003 |
| Optical Orientation | positive |
| Pleochroism | |
| Dispersion | 79° |
| Used Literature | |
| [Broul.etal:1981]Title: Solubility in organic two component systems Author: Broul M., Nyvlt J.; Soehnel O.  [Dana:1951]Title: Dana's System of Mineralogy [Dana:1951]Title: Dana's System of MineralogyAuthor: Dana J.D.  [Steiger.etal:2011a]Title: Decomposition reactions of magnesium sulfate hydrates and phase equilibria in the MgSO4-H2O and Na+-Mg2+-Cl--SO42--H2O systems with implications for Mars [Steiger.etal:2011a]Title: Decomposition reactions of magnesium sulfate hydrates and phase equilibria in the MgSO4-H2O and Na+-Mg2+-Cl--SO42--H2O systems with implications for MarsAuthor: Steiger, Michael; Linnow, Kirsten; Ehrhardt, Dorothee; Rohde, Mandy 
| |
Solubility[edit]
Bischofite has got a high solubility in water (5.75 mol/kg at 20 °C). The solubility of bischofite and other phases of the system MgCl2-H2O is shown in Fig. 1.
Hygroscopicity[edit]
Figure 2 includes the phase diagram of the system MgCl2-H2O. Next to the hexahydrate bischofite there are two more stable phases within the regarded temperature range. The octa- and the dodecahydrate are relevant at lower temperatures.
| 0°C | 10°C | 20°C | 30°C | 40°C | 50°C |
| 34.1%r.h. | 33.7%r.h. | 33.1%r.h. | 32.4%r.h. | 31.5%r.h. | 30.5%r.h. |
Weblinks[edit]
- ↑ http://webmineral.com/data/Bischofite.shtml viewed on 29/07/2010
- ↑ http://www.mindat.org/min-681.html viewed on 29/07/2010
Literature[edit]
| [Broul.etal:1981] | Elsevier (eds.) Broul M., Nyvlt J.; Soehnel O. (1981): Solubility in organic two component systems, Elsevier |  |
| [Dana:1951] | Dana E.S. (eds.) Dana J.D. (1951): Dana's System of Mineralogy, 7, Wiley & Sons |  |
| [Steiger.etal:2011a] | Steiger, Michael; Linnow, Kirsten; Ehrhardt, Dorothee; Rohde, Mandy (2011): Decomposition reactions of magnesium sulfate hydrates and phase equilibria in the MgSO4-H2O and Na+-Mg2+-Cl--SO42--H2O systems with implications for Mars. In: Geochimica et Cosmochimica Act, 75 (12), 3600-3626, 10.1016/j.gca.2011.03.038, |  |
| [Steiger.etal:2014] | Steiger, Michael; Charola A. Elena; Sterflinger, Katja (2014): Weathering and Deterioration. In: Siegesmund S.; Snethlage R. (eds.): Stone in Architecture, Springer Verlag Berlin Heidelberg, 223-316, 10.1007/978-3-642-45155-3_4. |  |