Gypsum: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
SLeithaeuser (talk | contribs) No edit summary |
||
| Line 3: | Line 3: | ||
|Footnote = <ref>http://webmineral.com/data/Gypsum.shtml seen on 30.07.2010</ref><ref>http://www.mindat.org/min-1784.html seen on 30.07.2010</ref> | |Footnote = <ref>http://webmineral.com/data/Gypsum.shtml seen on 30.07.2010</ref><ref>http://www.mindat.org/min-1784.html seen on 30.07.2010</ref> | ||
|photo = [[File:SA101 1.jpeg|300px]] | |photo = [[File:SA101 1.jpeg|300px]] | ||
| | |Mineralogical_name = Gypsum, Selenite | ||
| | |Chemical_name = Calcium sulfate dihydrate | ||
| | |Trivial_name = Gypsite, Sulfate of Lime | ||
| | |Chemical_Formula = Ca[SO<sub>4</sub>]•2H<sub>2</sub>O | ||
|Hydratforms = Anhydrite (CaSO<sub>4</sub>)<br>Hemihydrate (CaSO<sub>4</sub>•0.5H<sub>2</sub>O) | |Hydratforms = Anhydrite (CaSO<sub>4</sub>)<br>Hemihydrate (CaSO<sub>4</sub>•0.5H<sub>2</sub>O) | ||
|Crystal_System = monoclinic | |Crystal_System = monoclinic | ||
| Line 17: | Line 17: | ||
|Transparency = transparent to opaque | |Transparency = transparent to opaque | ||
|Cleavage = perfect | |Cleavage = perfect | ||
| | |Crystal_habit = | ||
|Twinning = | |Twinning = | ||
| | |Refractive_indices = α = 1.519-1.521<br>β = 1.522-1.523<br>γ = 1.529-1.530 | ||
|Birefringence = Δ = 0.010 | |Birefringence = Δ = 0.010 | ||
| | |Optical_orientation = biaxial positive | ||
|Pleochroism = | |Pleochroism = | ||
|Dispersion = 58° | |Dispersion = 58° | ||
| | |Phase_transition = | ||
| | |chem behavior = | ||
|Comments = hardly soluble in water | |Comments = hardly soluble in water | ||
}} | }} | ||
Revision as of 17:24, 12 January 2012
<bibimport/>
| Gypsum[1][2] | |

| |
| Mineralogical name | {{{mineralogical_Name}}} |
| Chemical name | {{{chemical_Name}}} |
| Trivial name | {{{Trivial_Name}}} |
| Chemical formula | {{{chemical_Formula}}} |
| Other forms | Anhydrite (CaSO4) Hemihydrate (CaSO4•0.5H2O) |
| Crystal system | monoclinic |
| Crystal structure | |
| Deliquescence humidity 20°C | |
| Solubility (g/l) at 20°C | 2.14 g/l |
| Density (g/cm³) | 2.2-2.4 g/cm³ |
| Molar volume | 74.69 cm3/mol |
| Molar weight | 172.17g /mol |
| Transparency | transparent to opaque |
| Cleavage | perfect |
| Crystal habit | {{{Crystal_Habit}}} |
| Twinning | |
| Phase transition | {{{Phase_Transition}}} |
| Chemical behavior | {{{chemBehavior}}} |
| Comments | hardly soluble in water |
| Crystal Optics | |
| Refractive Indices | {{{Refractive_Indices}}} |
| Birefringence | Δ = 0.010 |
| Optical Orientation | {{{optical_Orientation}}} |
| Pleochroism | |
| Dispersion | 58° |
| Used Literature | |
| {{{Literature}}} | |
Authors: Hans-Jürgen Schwarz , Nils Mainusch, Tim Müller
back to Sulfate
Calciumsulfate and Gipsum[edit]
Solubility properties[edit]

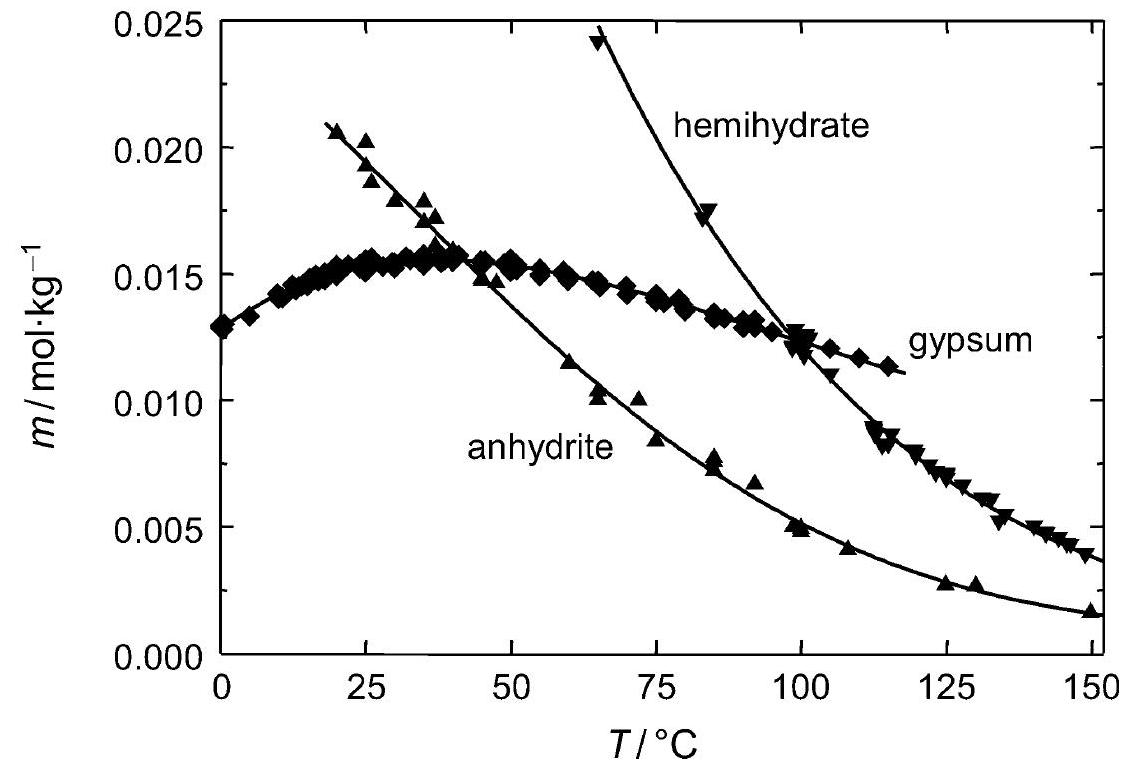
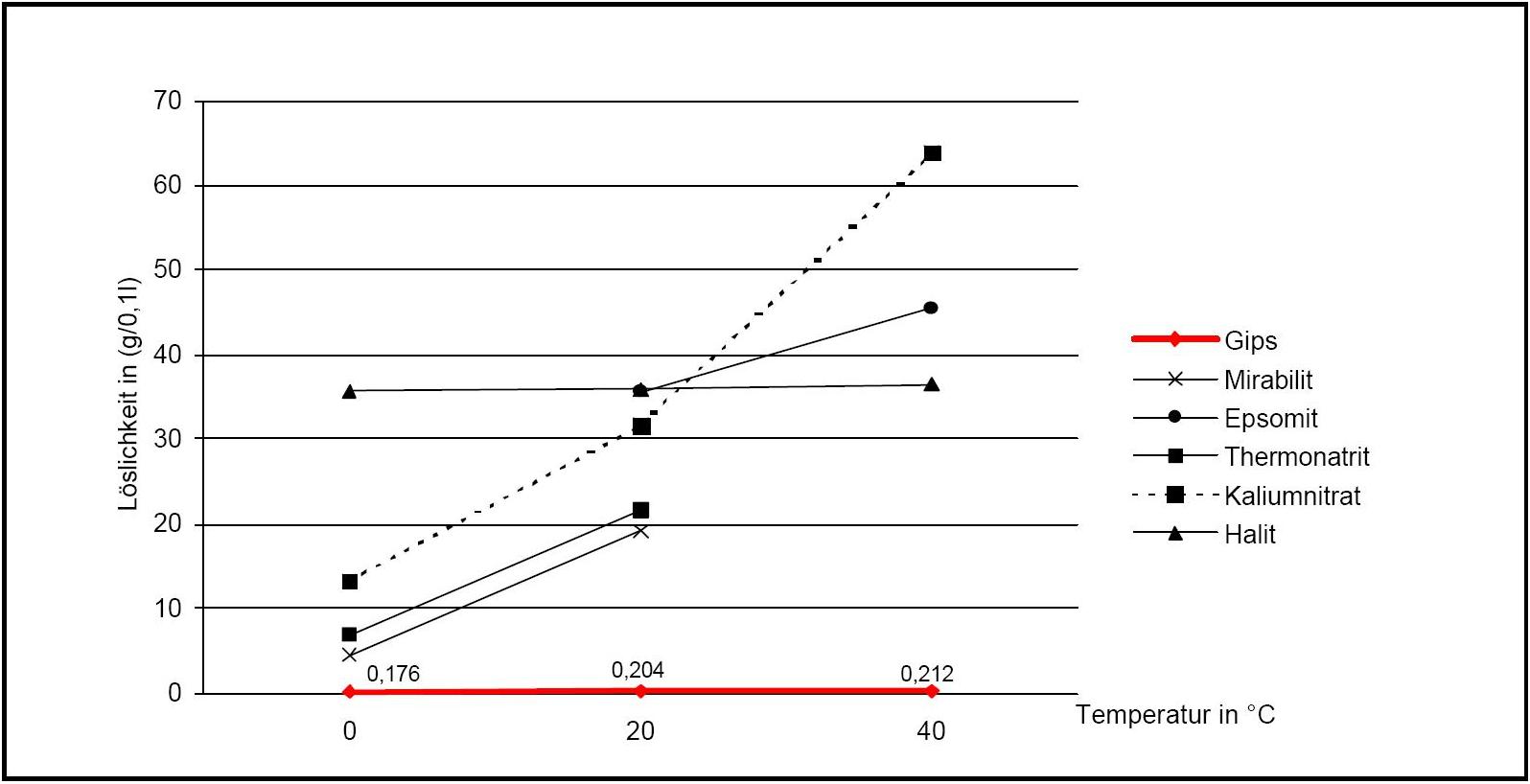
Figure 2:Solubility of gipsum compared with other salte (after [Stark.etal:1996]Title: Bauschädliche Salze
Author: Stark, Jochen; Stürmer, Sylvia
 )
)
Author: Stark, Jochen; Stürmer, Sylvia
 )
)
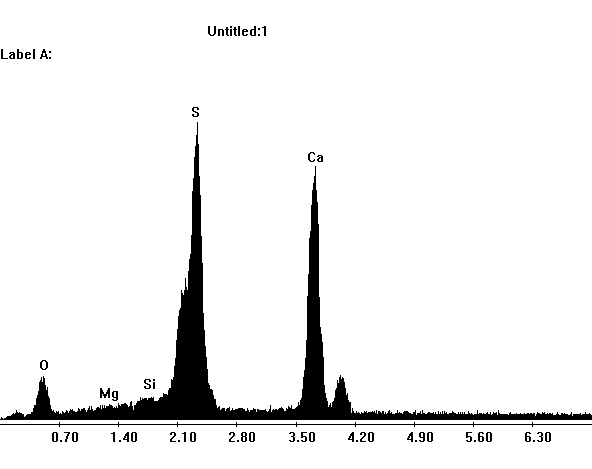
Photos of gypsum crystals and deterioration pattern caused by gypsum[edit]
On a object[edit]
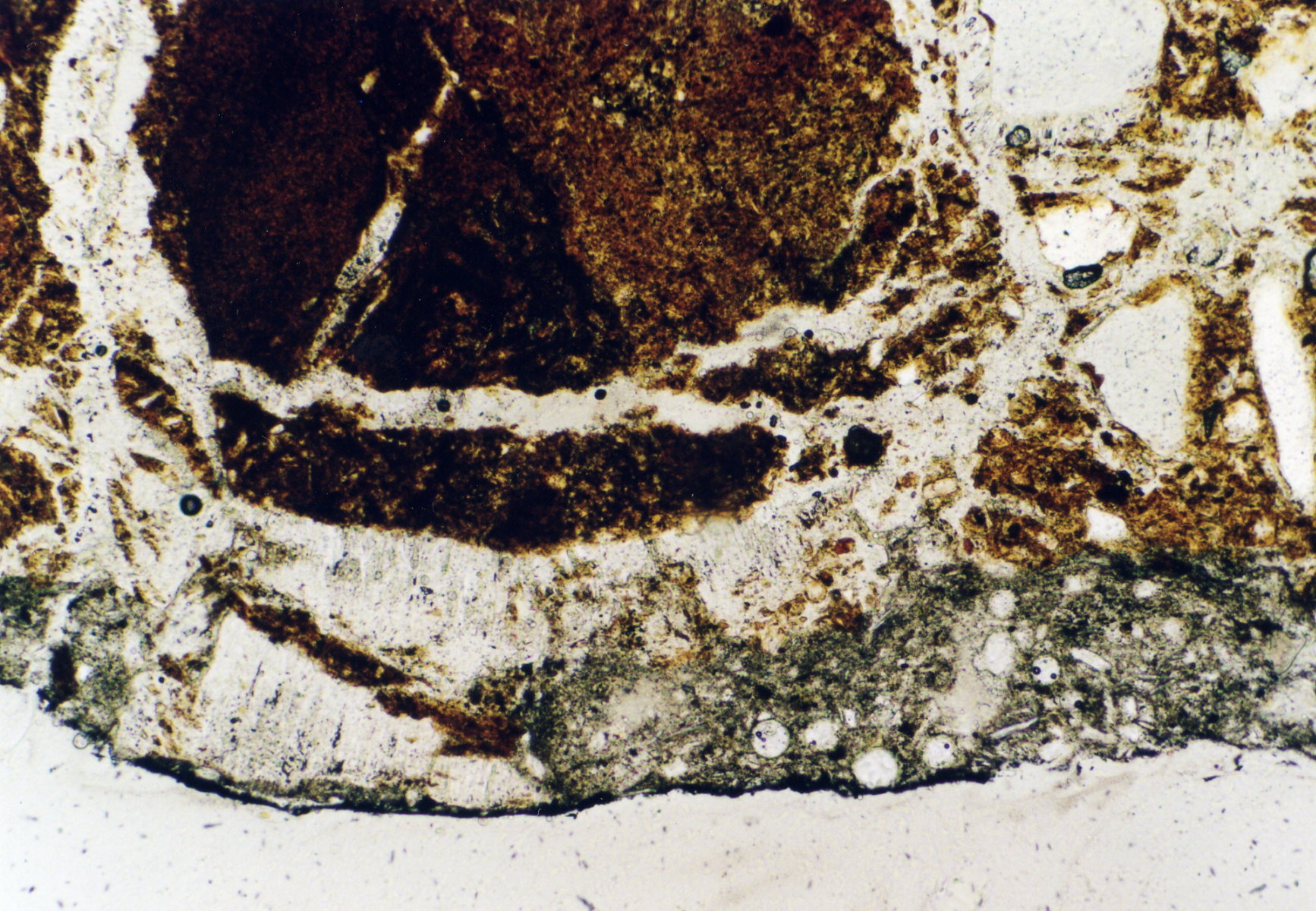
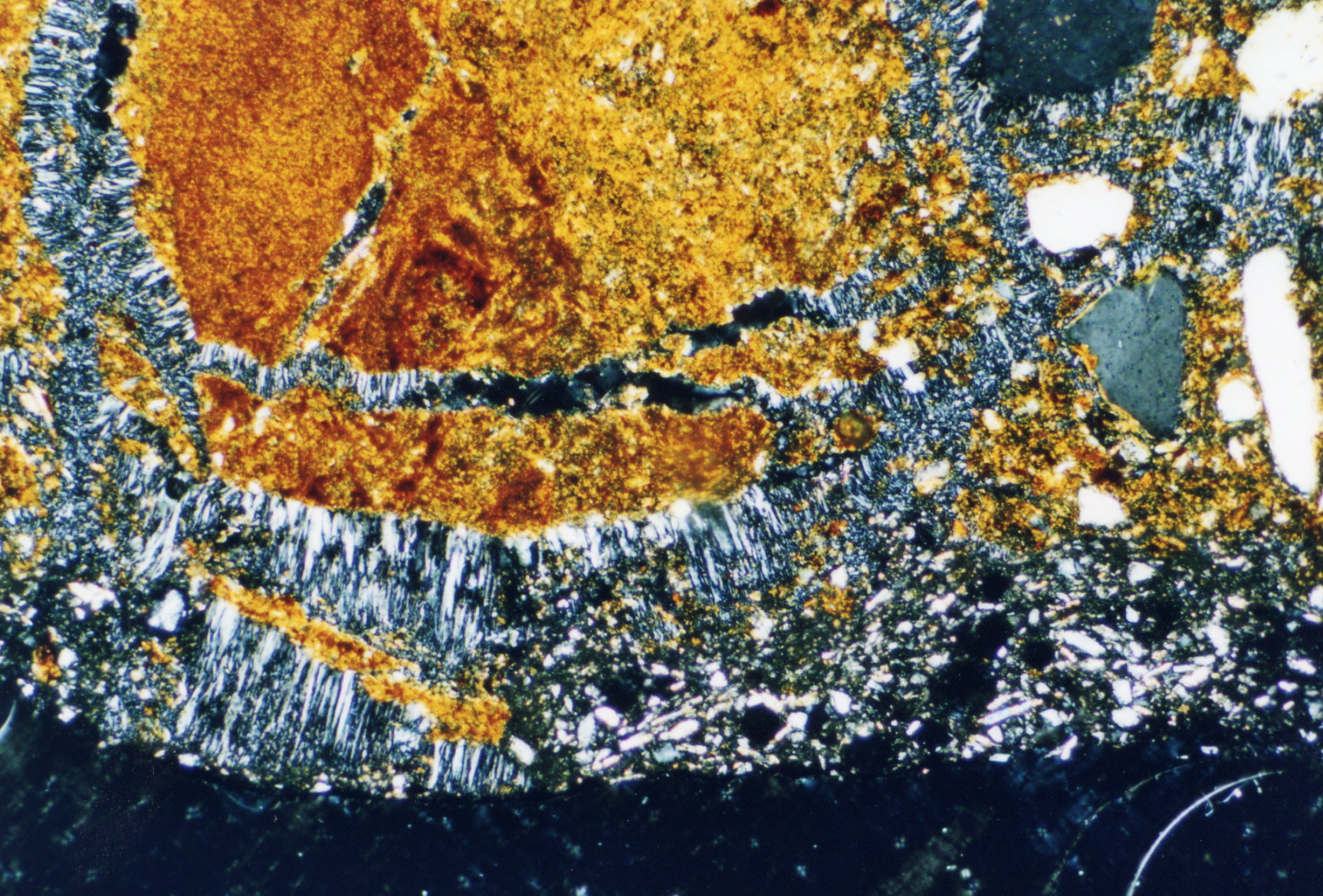
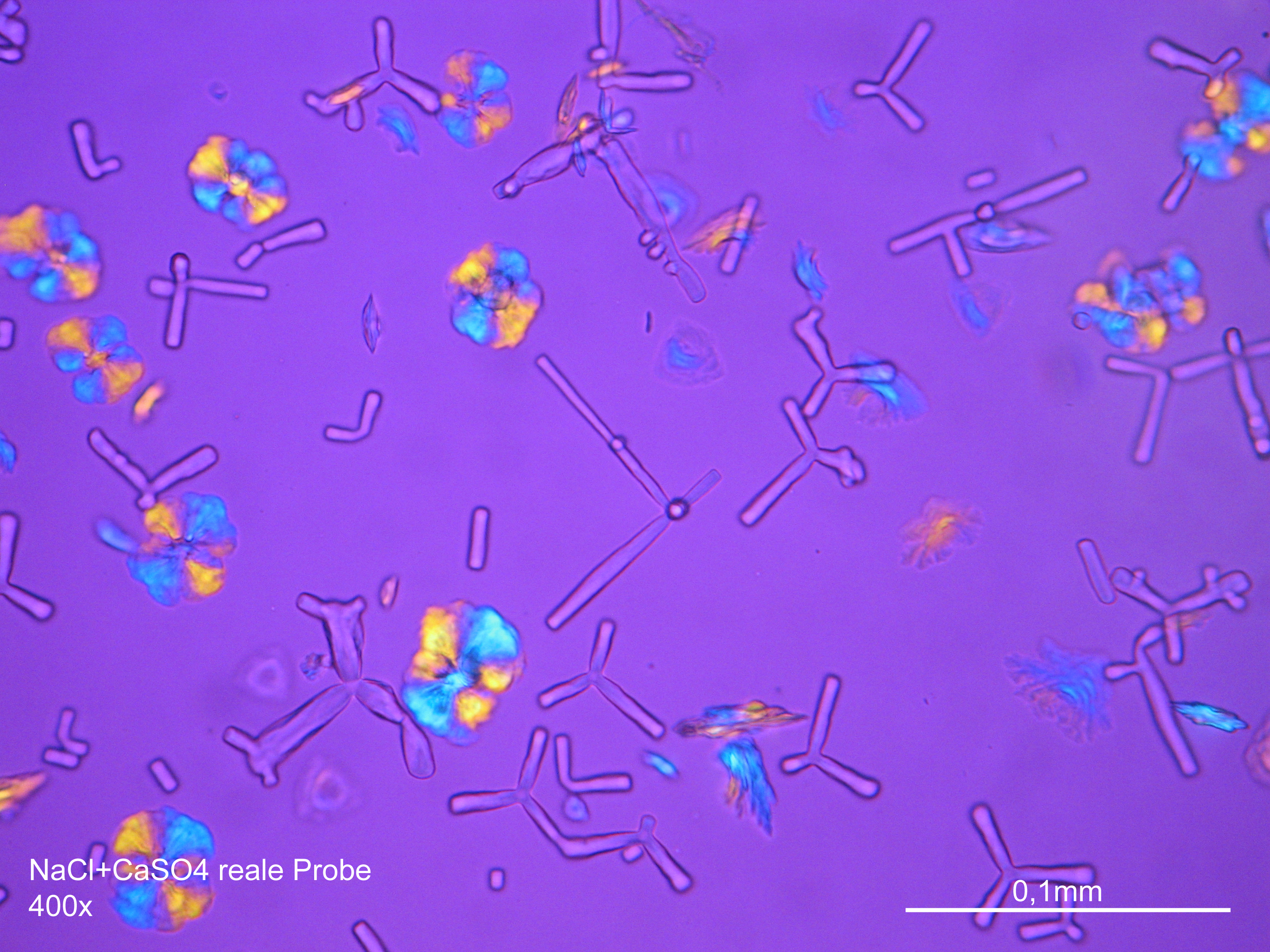
Under the polarising microscope[edit]
- In thin section of bricks
- Gypsum crystallized out of a solution in water on a glass slide
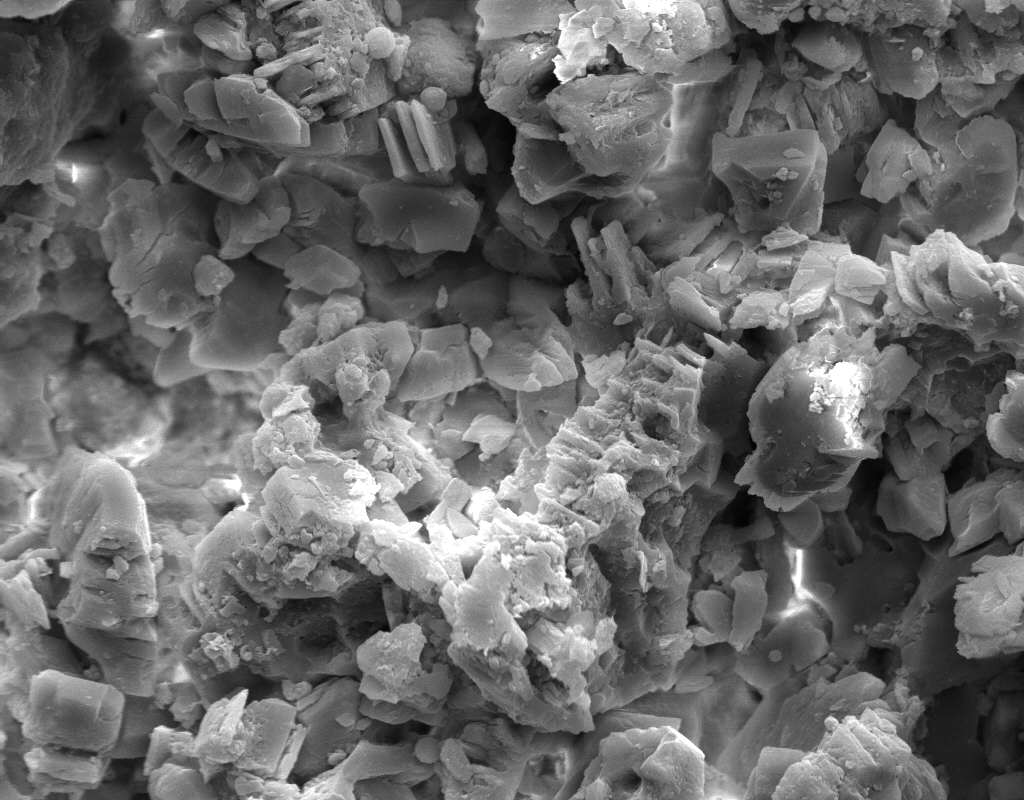
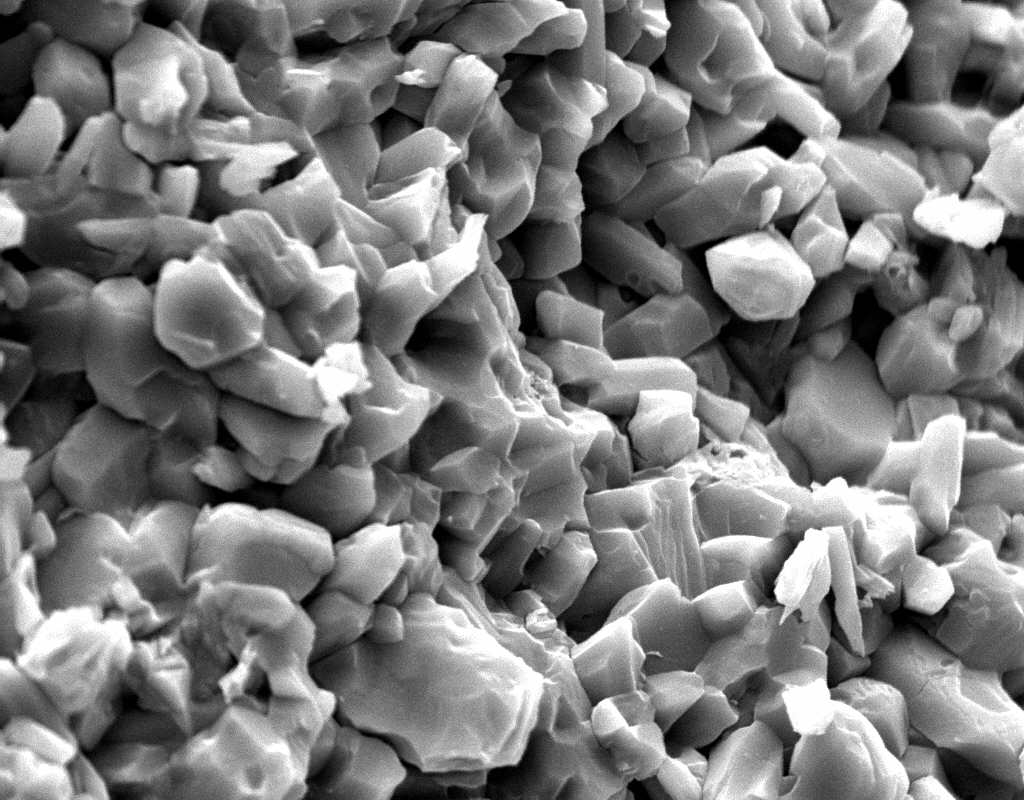
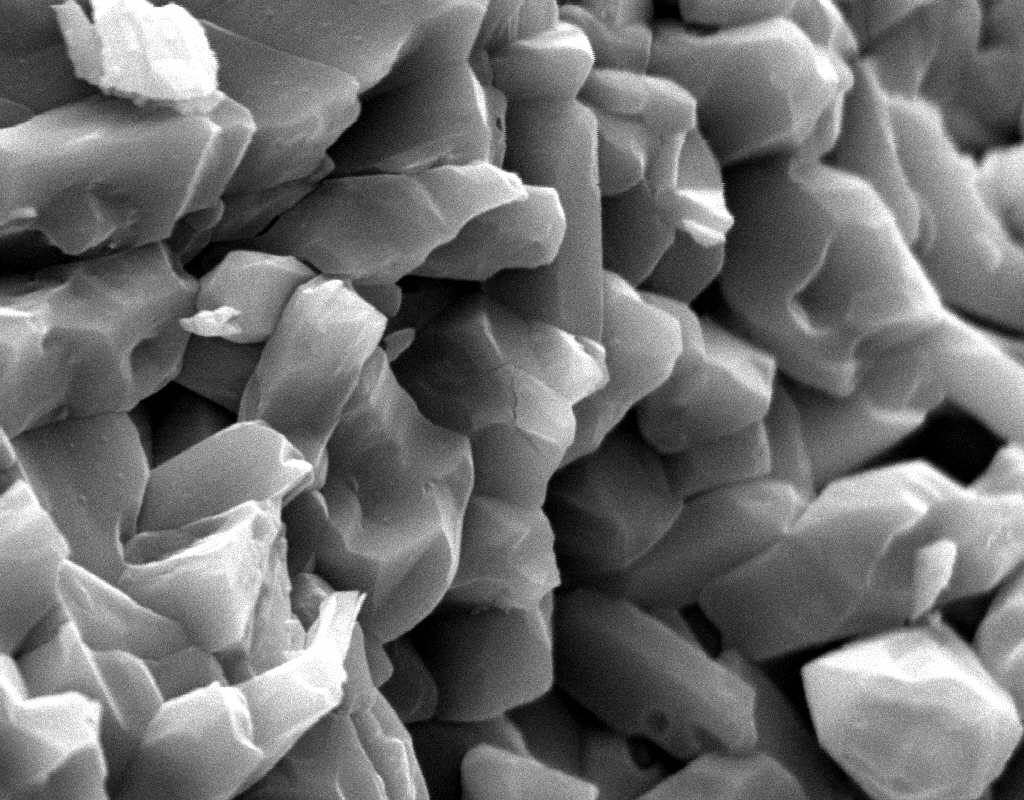
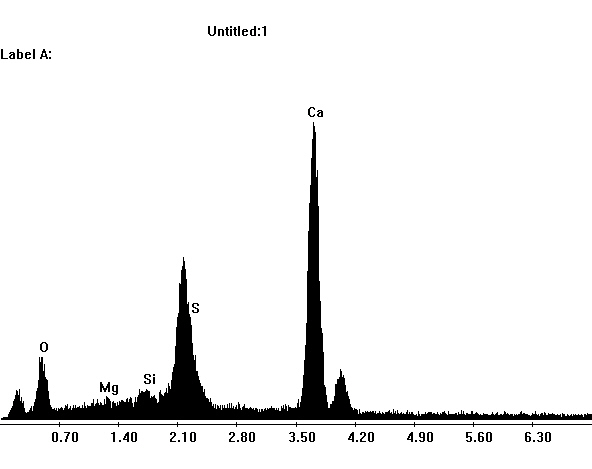
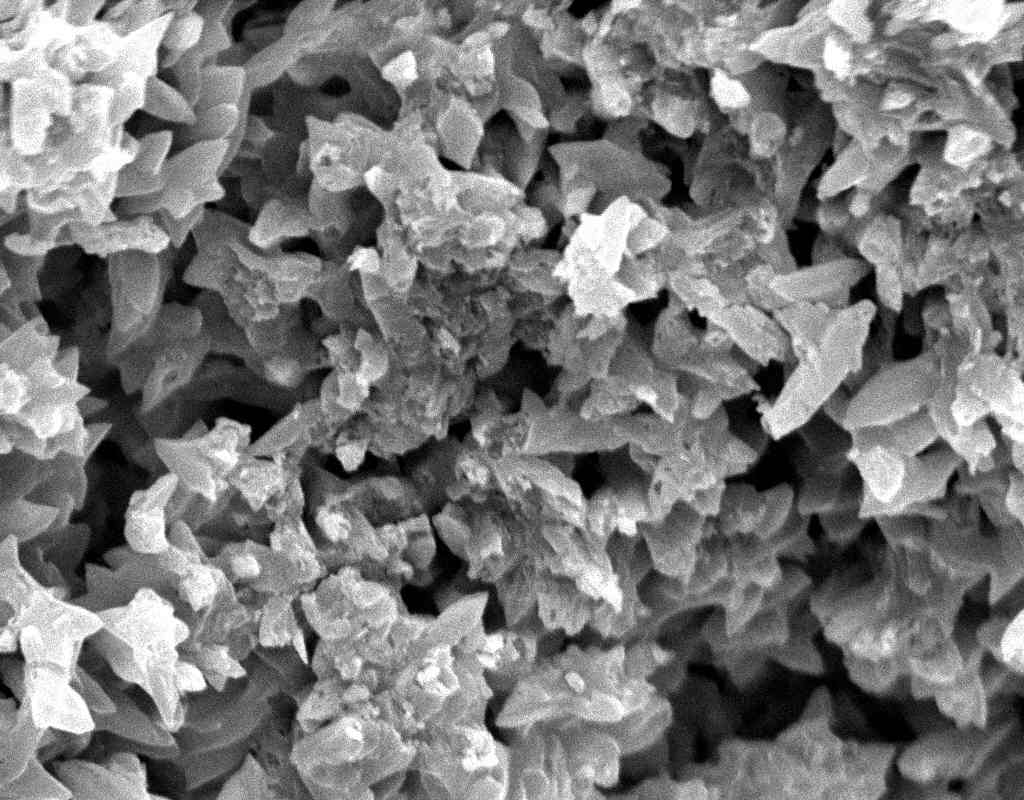
Under the Scanning Electron Microscope (SEM)[edit]
- In a SEM
Weblinks[edit]
- ↑ http://webmineral.com/data/Gypsum.shtml seen on 30.07.2010
- ↑ http://www.mindat.org/min-1784.html seen on 30.07.2010
Literatur[edit]
[Filter missing]