Gypsum: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
|||
| Line 214: | Line 214: | ||
<bibprint/> | <bibprint/> | ||
<references /> | <references /> | ||
[[Category:Gipsum]][[Category:Sulphate]][[Category:Salt]][[Category:InProgress]][[Category:Sulfate]] | [[Category:Gipsum]][[Category:Sulphate]][[Category:Salt]][[Category:InProgress]][[Category:Sulfate]] | ||
Revision as of 12:17, 24 January 2012
<bibimport/>
| Gypsum[1][2] | |

| |
| Mineralogical name | Gypsum, Selenite |
| Chemical name | Calcium sulfate dihydrate |
| Trivial name | {{{Trivial_Name}}} |
| Chemical formula | {{{chemical_Formula}}} |
| Other forms | Anhydrite (CaSO4) Hemihydrate (CaSO4•0.5H2O) |
| Crystal system | monoclinic |
| Crystal structure | |
| Deliquescence humidity 20°C | |
| Solubility (g/l) at 20°C | 2.14 g/l |
| Density (g/cm³) | 2.2-2.4 g/cm³ |
| Molar volume | 74.69 cm3/mol |
| Molar weight | 172.17g /mol |
| Transparency | transparent to opaque |
| Cleavage | perfect |
| Crystal habit | {{{Crystal_Habit}}} |
| Twinning | |
| Phase transition | {{{Phase_Transition}}} |
| Chemical behavior | {{{chemBehavior}}} |
| Comments | hardly soluble in water |
| Crystal Optics | |
| Refractive Indices | {{{Refractive_Indices}}} |
| Birefringence | Δ = 0.010 |
| Optical Orientation | {{{optical_Orientation}}} |
| Pleochroism | |
| Dispersion | 58° |
| Used Literature | |
| {{{Literature}}} | |
Authors: Hans-Jürgen Schwarz , Nils Mainusch, Tim Müller
back to Sulfate
Calciumsulfate and Gipsum[edit]
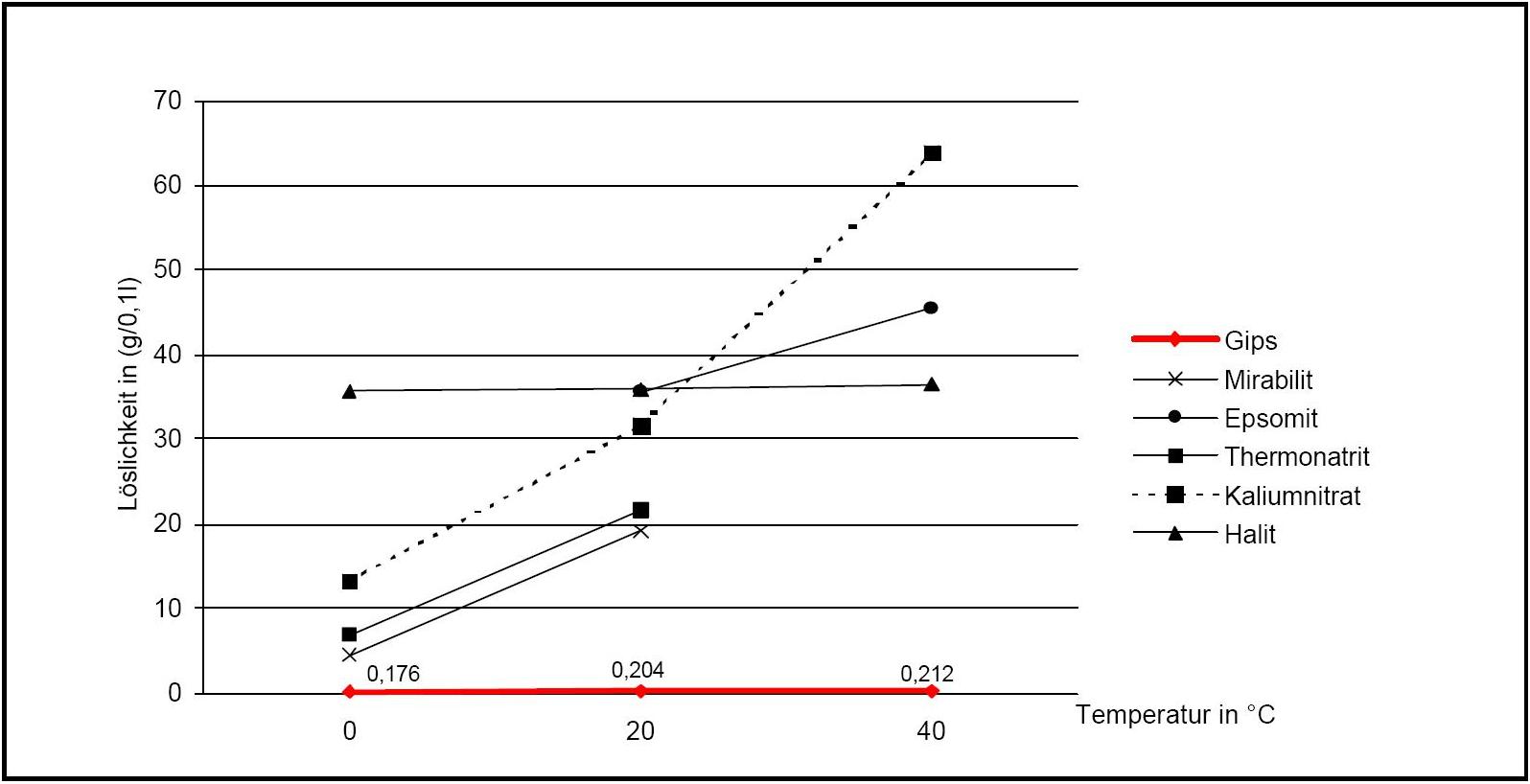
Solubility properties[edit]

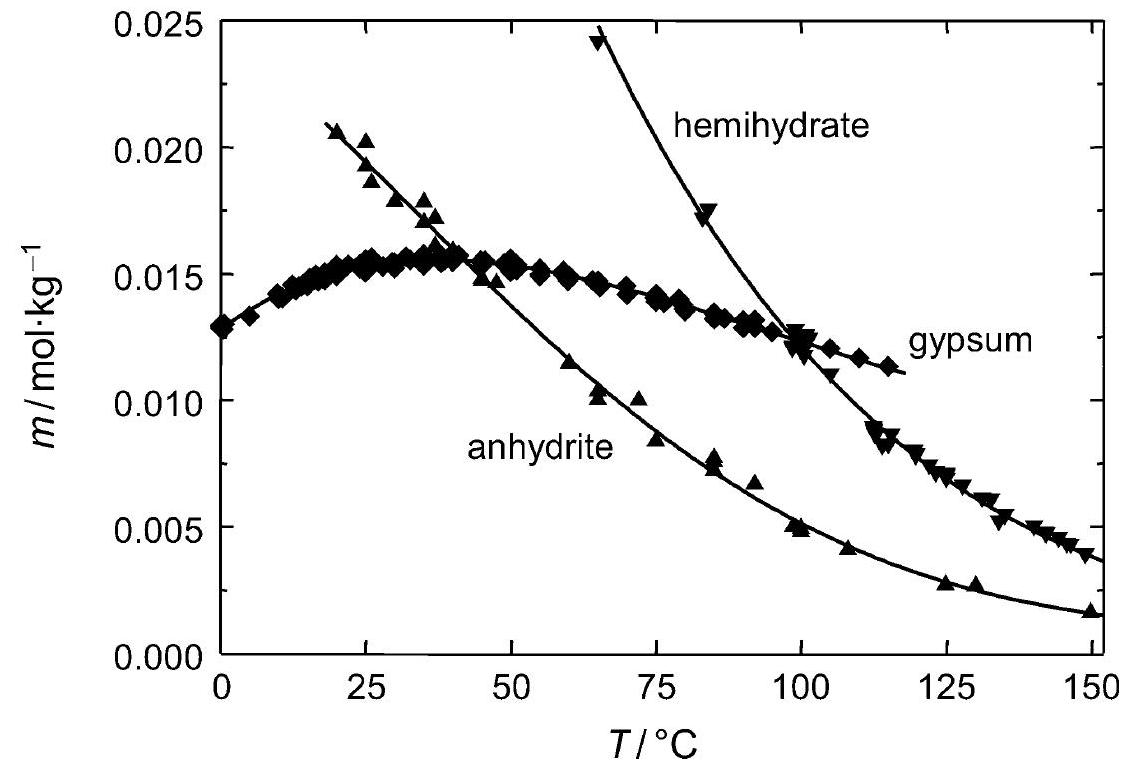
Figure 2:Solubility of gipsum compared with other salte (after [Stark.etal:1996]Title: Bauschädliche Salze
Author: Stark, Jochen; Stürmer, Sylvia
 )
)
Author: Stark, Jochen; Stürmer, Sylvia
 )
)
Photos of gypsum crystals and deterioration pattern caused by gypsum[edit]
On an object[edit]
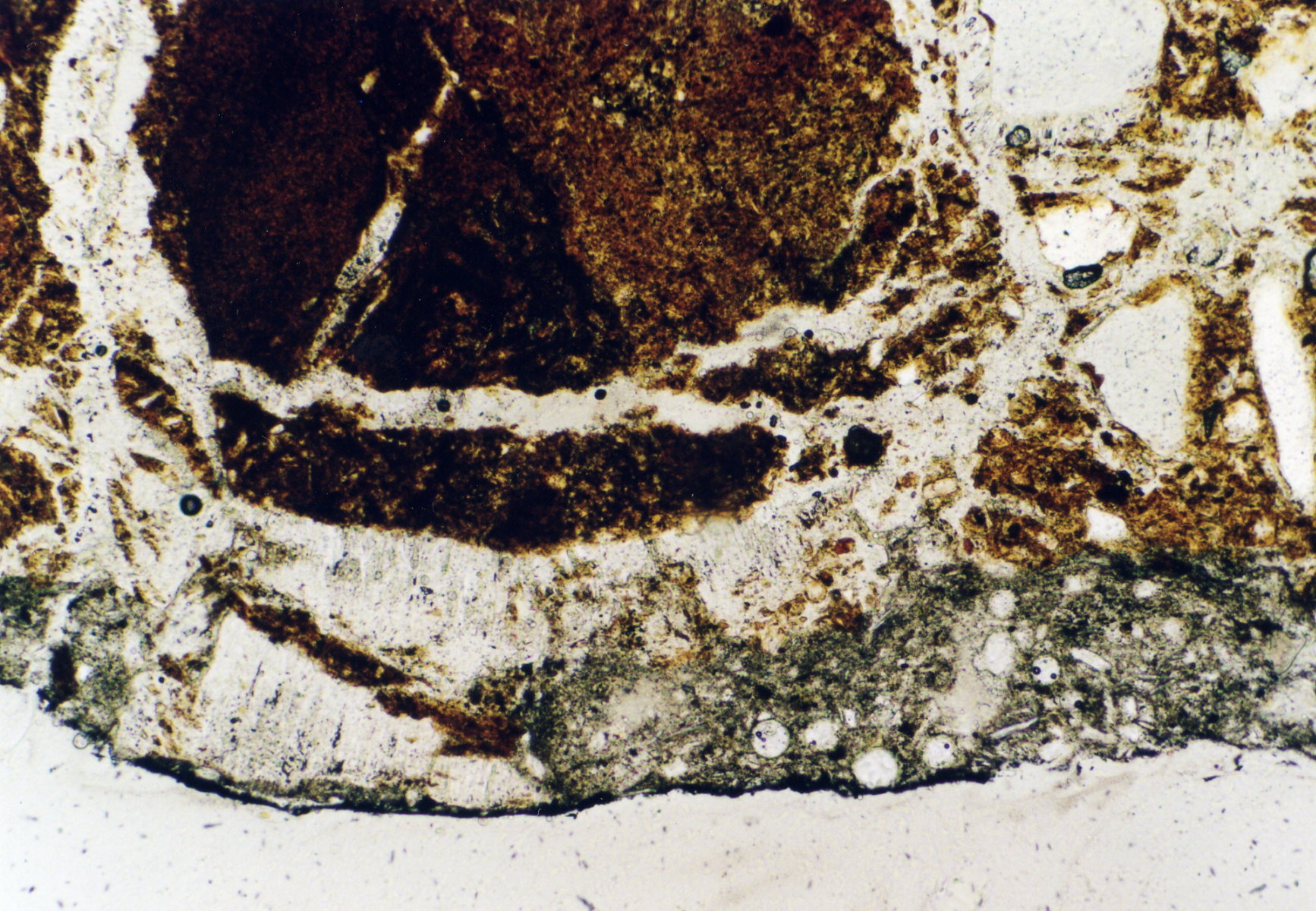
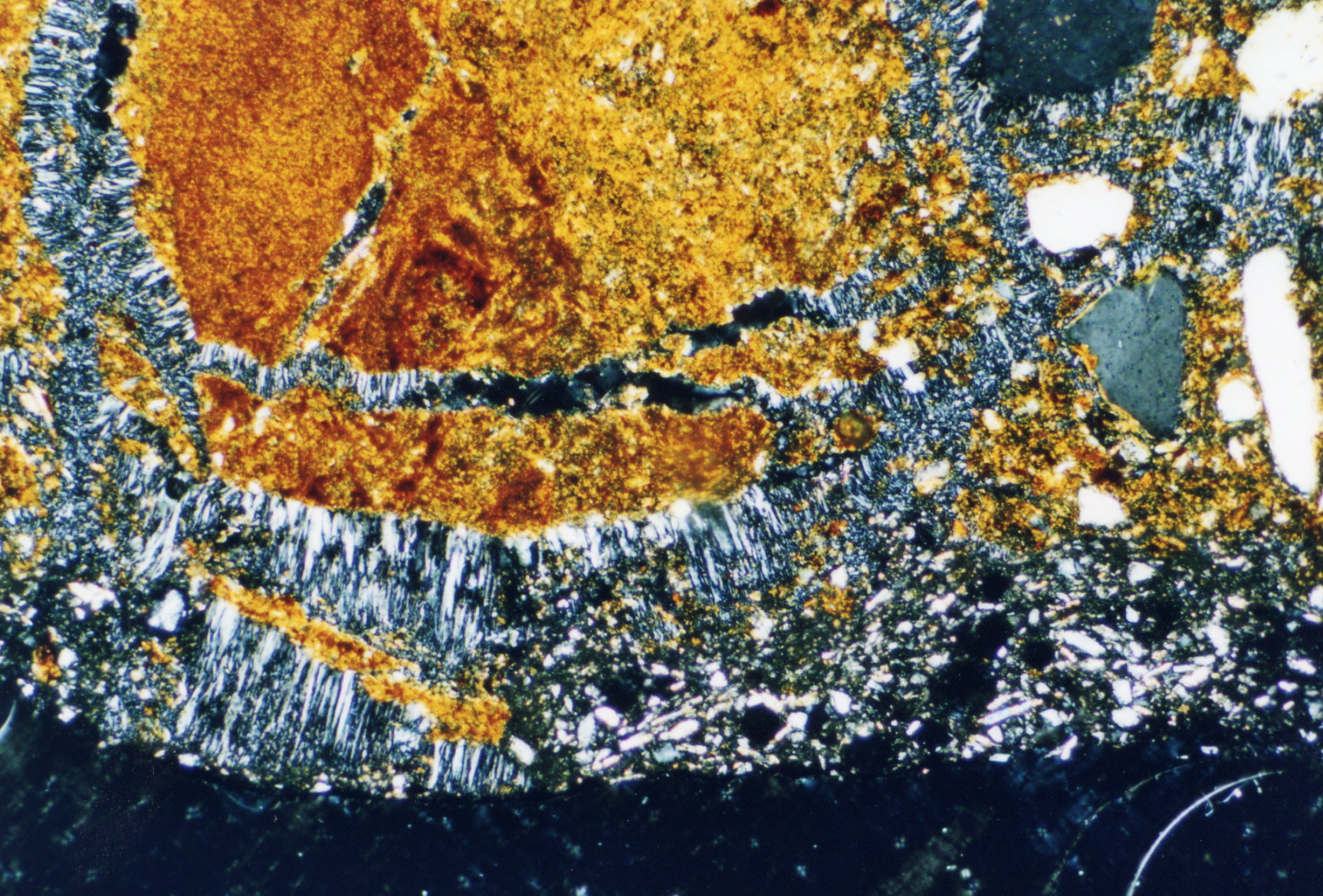
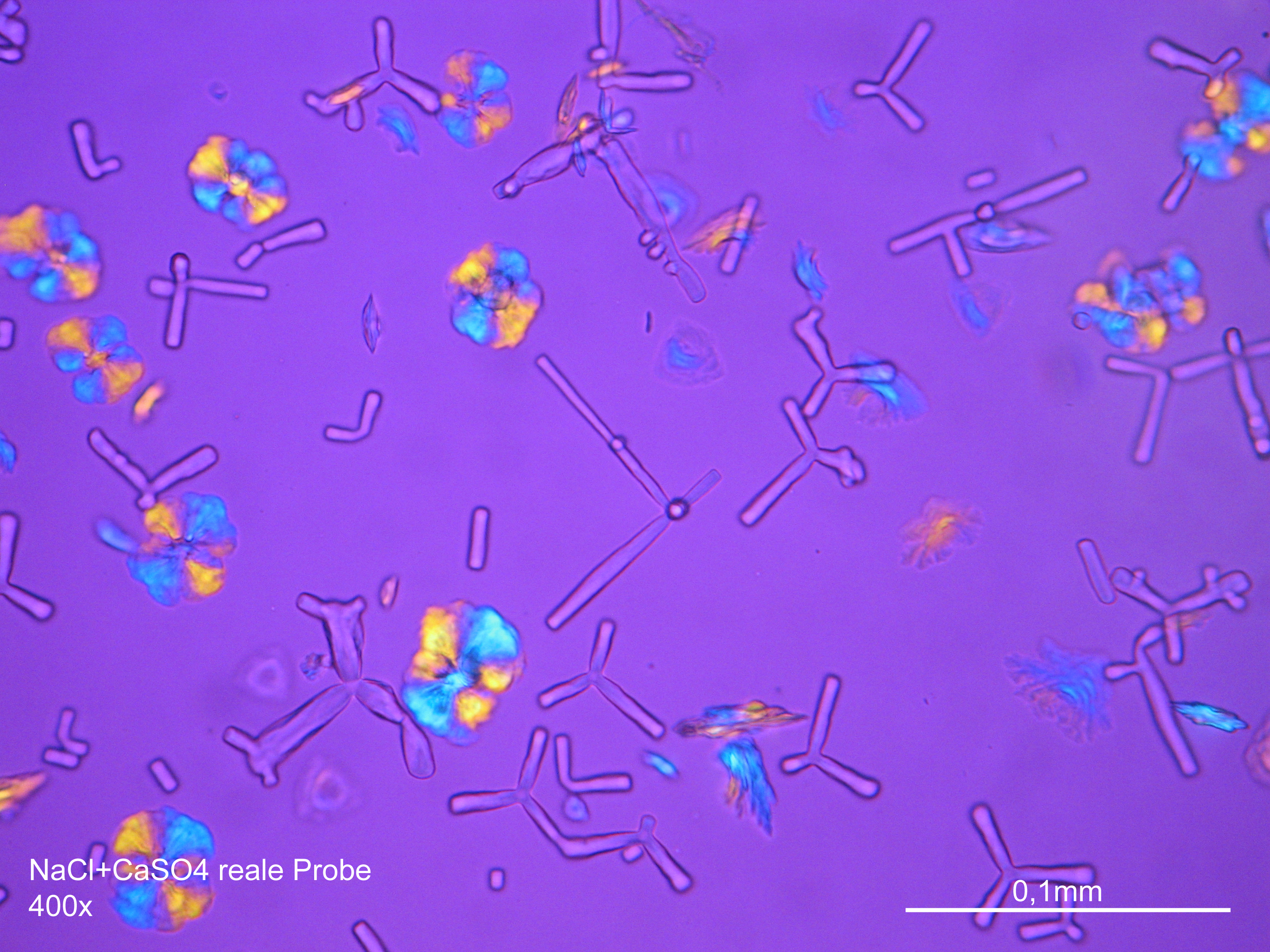
Under the polarising microscope[edit]
- In thin section of bricks
- Gypsum crystallized out of a solution in water on a glass slide
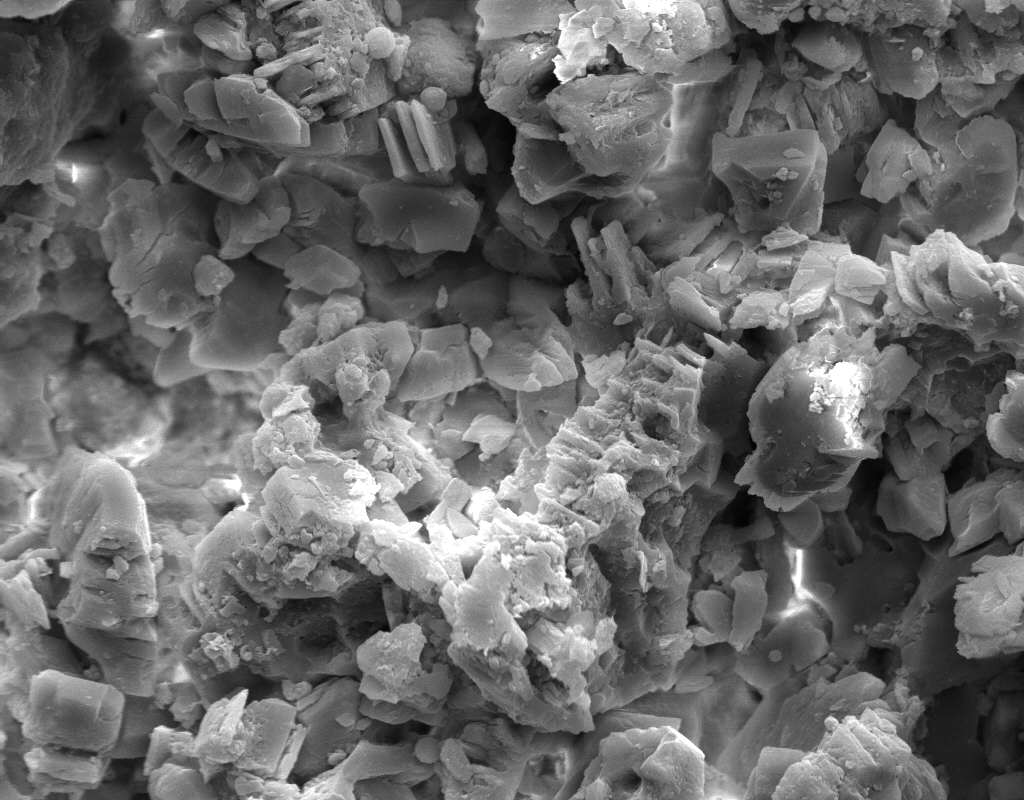
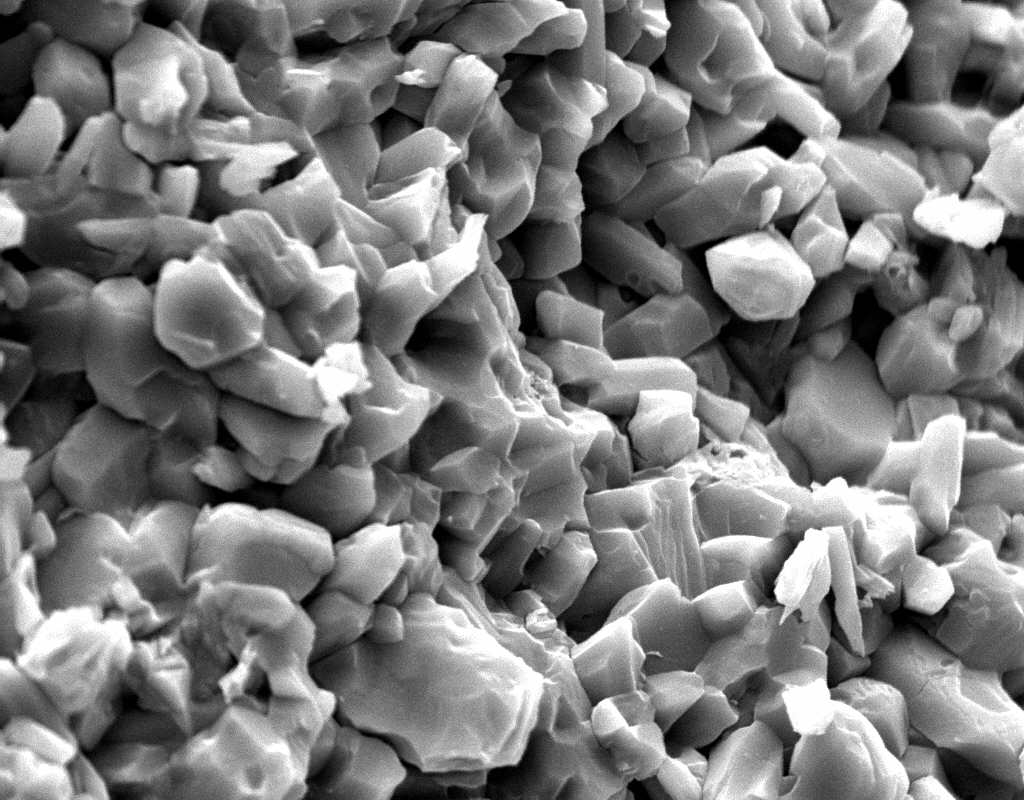
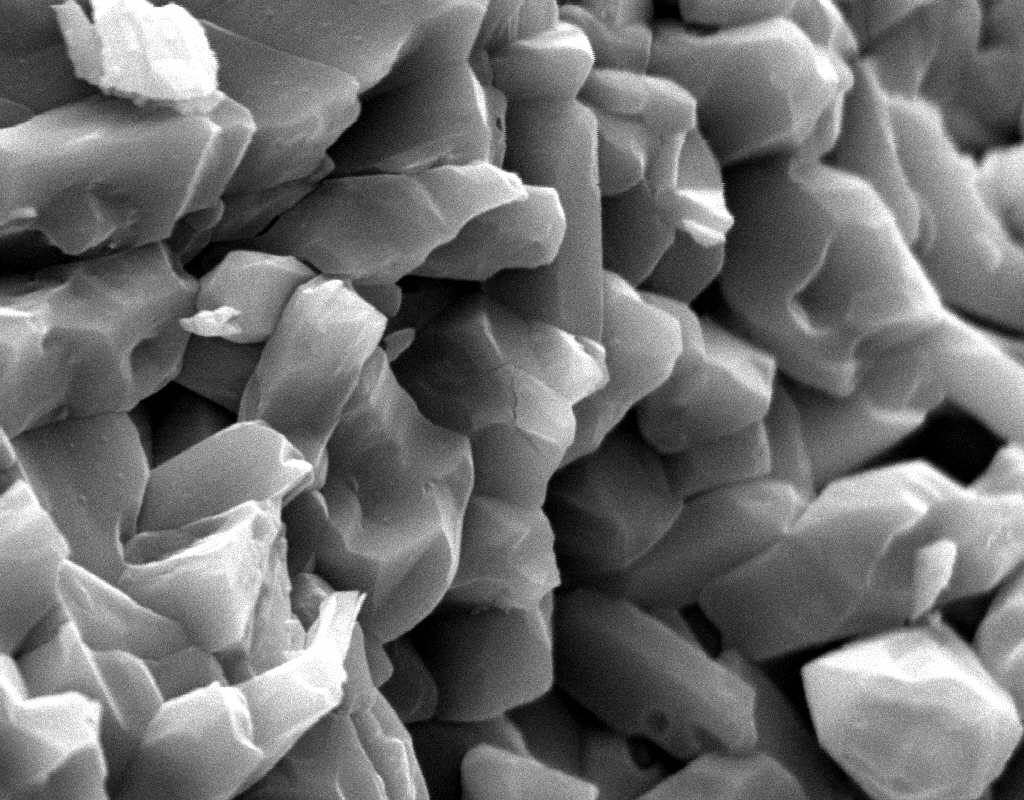
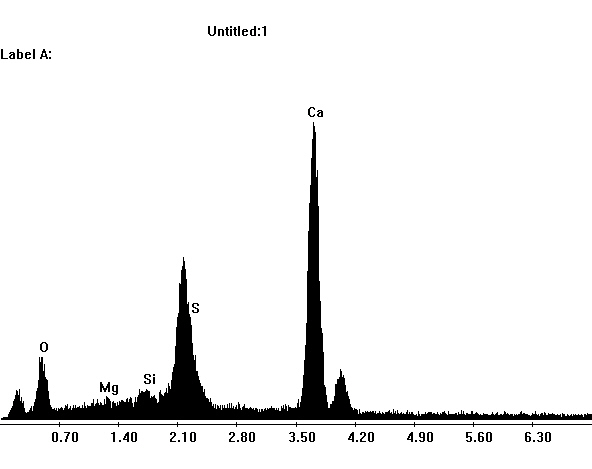
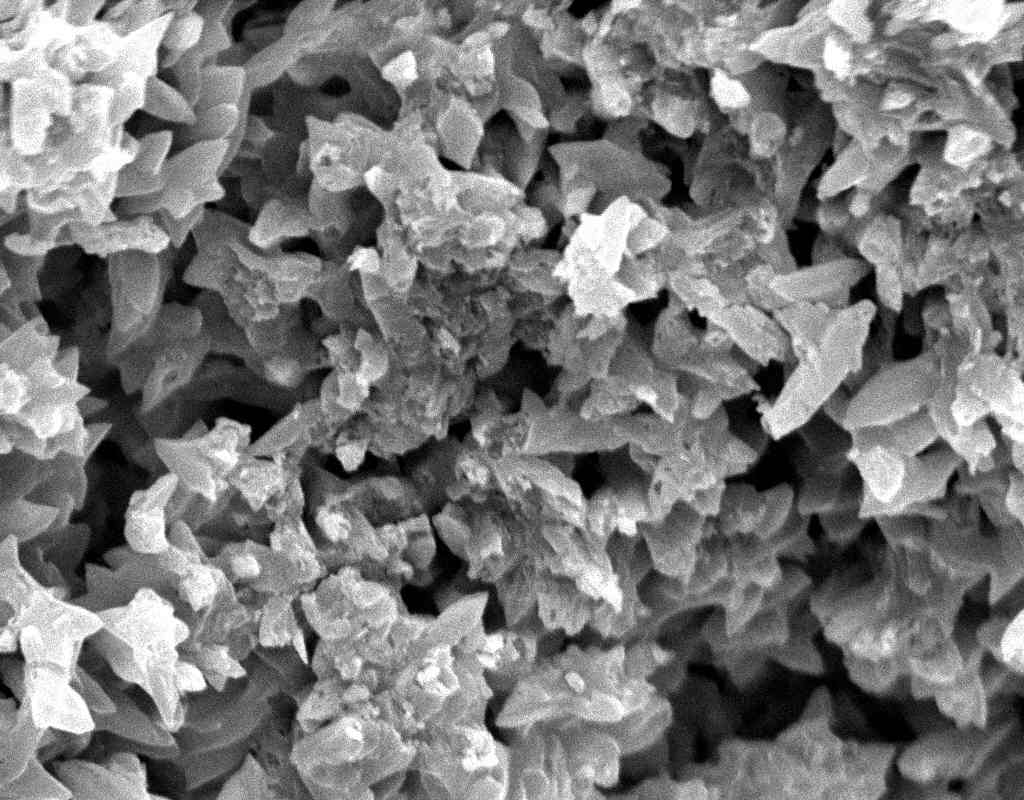
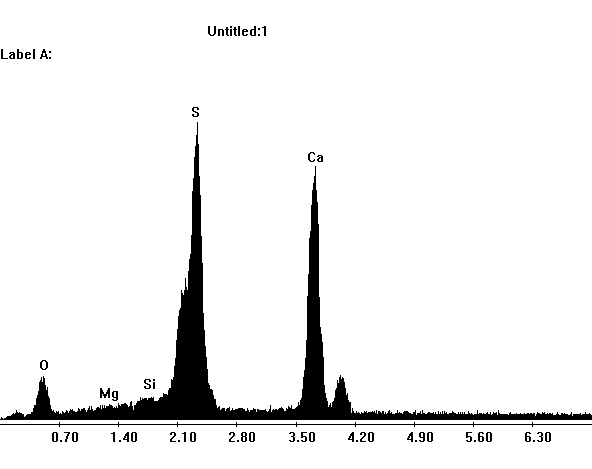
Under the Scanning Electron Microscope (SEM)[edit]
- In a SEM
Weblinks[edit]
- ↑ http://webmineral.com/data/Gypsum.shtml seen on 30.07.2010
- ↑ http://www.mindat.org/min-1784.html seen on 30.07.2010
Literatur[edit]
[Filter missing]