Gypsum: Difference between revisions
No edit summary |
|||
| (45 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
< | Authors: [[user:Hschwarz|Hans-Jürgen Schwarz ]], [[user:NMainusch|Nils Mainusch]], [[user:TMueller|Tim Müller]] | ||
<br>English Translation by Sandra Leithäuser | |||
<br>back to [[Sulfate]] | |||
{{Infobox_Salt | {{Infobox_Salt | ||
|Footnote = <ref>http://webmineral.com/data/Gypsum.shtml seen on 30.07.2010</ref><ref>http://www.mindat.org/min-1784.html seen on 30.07.2010</ref> | |Footnote = <ref>http://webmineral.com/data/Gypsum.shtml seen on 30.07.2010</ref><ref>http://www.mindat.org/min-1784.html seen on 30.07.2010</ref> | ||
|photo = [[File:SA101 1.jpeg|300px]] | |photo = [[File:SA101 1.jpeg|300px]] | ||
|mineralogical_Name = Gypsum | |mineralogical_Name = Gypsum | ||
|chemical_Name = Calcium sulfate dihydrate | |chemical_Name = Calcium sulfate dihydrate | ||
| | |Trivial_Name = Alabaster, Satin Spar, Selenite, | ||
| | |chemical_Formula = CaSO<sub>4</sub>•2H<sub>2</sub>O | ||
|Hydratforms = | |Hydratforms = CaSO<sub>4</sub> ([[Anhydrite]])<br> CaSO<sub>4</sub>•0.5H<sub>2</sub>O ([[Bassanite]]) | ||
|Crystal_System = monoclinic | |Crystal_System = monoclinic | ||
|Crystal_Structure = | |Crystal_Structure = | ||
|Deliqueszenzhumidity = | |Deliqueszenzhumidity = > 99% RH at 20°C | ||
|Solubility = 2.14 g/l | |Solubility = 2.14 g/l | ||
|Density = 2. | |Density = 2.31 g/cm³ | ||
|MolVolume = 74.69 cm<sup>3</sup>/mol | |MolVolume = 74.69 cm<sup>3</sup>/mol | ||
|Molweight = 172.17g /mol | |Molweight = 172.17g /mol | ||
|Transparency = transparent to opaque | |Transparency = transparent to opaque | ||
|Cleavage = perfect | |Cleavage = perfect, clearly visible formation of fibre | ||
| | |Crystal_Habit = flat, prismatic, needle- like crystal, granular, massive aggregate | ||
|Twinning = | |Twinning = very common | ||
| | |Refractive_Indices = α = 1.5207<br>β = 1.5230<br>γ = 1.5299 | ||
|Birefringence = Δ = 0. | |Birefringence = Δ = 0.0092 | ||
| | |optical_Orientation = biaxial positive | ||
|Pleochroism = | |Pleochroism = colorless | ||
|Dispersion = 58° | |Dispersion = 58° | ||
| | |Phase_Transition = | ||
| | |chemBehavior = hardly soluble in water | ||
|Comments = | |Comments = | ||
|Literature =<bib id="Robie.etal:1978"/> <bib id="Dana:1951"/> | |||
}} | }} | ||
=Calcium sulfate and gypsum = | |||
== Abstract == | == Abstract == | ||
The article discusses the system CaSO<sub>4</sub>-H<sub>2</sub>O, in reference to gypsum. Gypsum is one of the most important salts in the deterioration of building materials and, particularly, wall-paintings. Objects exposed to exterior conditions where air pollution is present are the most prone to damage from gypsum. The appearance and mechanism of the damage, as well as the examination methods are described. Images, microphotographs and examples from practical experiences illustrate the subject.<br> | |||
<br> | <br> | ||
== | == Introduction == | ||
Gypsum is one of the most common salts causing deterioration of inorganic porous building materials. It is present on most exterior exposed surfaces and even in interior environments, under different shapes and inducing various deterioration patterns. <br> | |||
<br> | |||
<br> | <br> | ||
Gypsum, one of the most prevalent minerals, forms by precipitation from aqueous solution at temperatures under approximately 40°C. When the solution reaches higher temperatures (> 60°C) anhydrite precipitates out. Calcium sulfate and calcium sulfate dihydrate are often present in rocks. Hemihydrate does not occur naturally.<br> | |||
Gypsum occurs naturally in salt deposits and deserts, where "desert rose" crystals form in combination with quartz inclusions. In natural salt deposits, gypsum and anhydrite sometimes form a caprock, i.e., a massive layer of material covering the deposit. | |||
Synthetic gypsum is produced in coal-fired power plants, as a by-product of flue-gas desulfurization. | |||
== | == Origin and formation of gypsum on monuments == | ||
On monuments made from porous inorganic building materials, particularly in urban environment where anthropogenic air pollutants are present, such as sulfur oxides (SO<sub>x</sub>) that eventually convert to sulfuric acid in the presence of moisture, the reaction of these gases with any calcium carbonate present in limestone, sandstone, mortars, renders, results in the formation of gypsum. The reaction taking place can be simplified as follows: | |||
CaCO<sub>3</sub> + H<sub>2</sub>SO<sub>4</sub> → CaSO<sub>4</sub> + H<sub>2</sub>O + CO<sub>2</sub> | CaCO<sub>3</sub> + H<sub>2</sub>SO<sub>4</sub> → CaSO<sub>4</sub> + H<sub>2</sub>O + CO<sub>2</sub> | ||
It is to be remembered that gypsum can be a major component of some mortars, plasters and even some building stones (e.g., selenite, used for the base of the Garrisenda Tower in Bologna, Italy) thus being an integral part of the monuments fabric. | |||
<br> | <br> | ||
== | == Damage potential and weathering activity == | ||
=== Solubility properties === | === Solubility properties === | ||
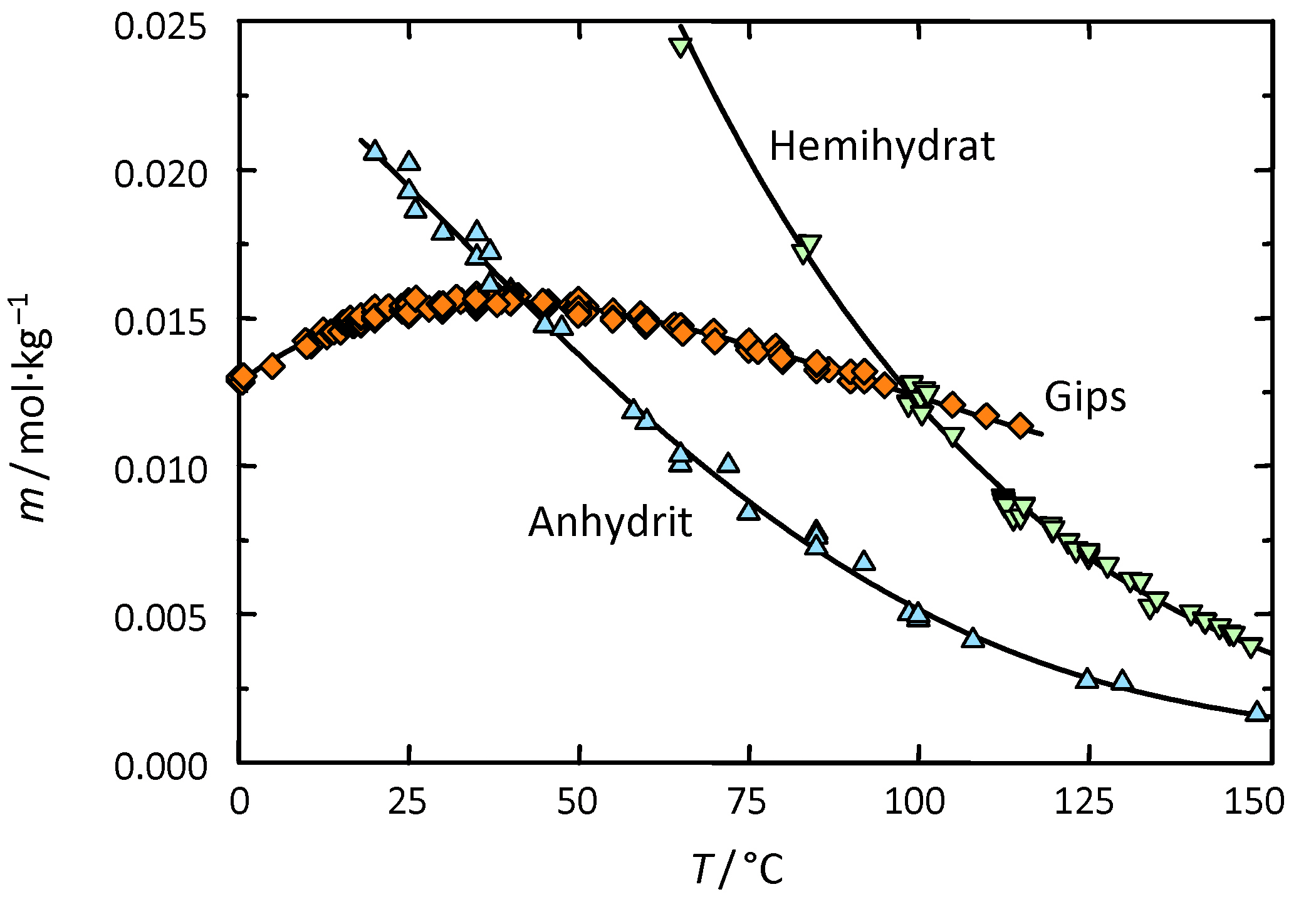
[[file:CaSO4-Steiger-1.jpg|thumb|350px|right|'''Figure1:''' Solubility of CaSO4 in water (diagram: Michael Steiger)]] | |||
Gypsum belongs to the group of salts with a low solubility in aqueous solution and therefore is less mobile than the more soluble ones. However, when other ions are present, its solubility can be significantly increased. For example, when halite, NaCl, is present, the solubility of gypsum can be increased by a factor four depending on the concentration ratio of the two salts. <br> | |||
[[file:Loeslichkeit Gips 02.JPG|thumb|right|350px|'''Figure 2:'''Solubility of gypsum compared with other salts (after <bib id="Stark.etal:1996"/>)]] | |||
[[file:Loeslichkeit Gips 02.JPG|thumb| | |||
=== Hydration behavior === | |||
< | The system CaSO<sub>4 </sub>–H<sub>2</sub>O: <br> Calcium sulfate can appear in three different hydrate phases: | ||
*Anhydrite (CaSO<sub>4</sub>)- the above mentioned anhydrous form. | |||
*Bassanite (hemihydrate) (CaSO<sub>4</sub>•0.5H<sub>2</sub>O)- a metastable form. | |||
*Gypsum (CaSO<sub>4</sub>•2H<sub>2</sub>O)- calcium sulfate dihydrate. | |||
=== | Anhydrite exists in different varieties with different chemical properties, such as different solubilities in water, depending on the conditions of its formation. The same applies to bassanite, the hemihydrate. <br> | ||
The transition temperature in aqueous solution for the gypsum-bassanite (dihydrate to hemihydrate) is in the 40°C-66°C range. Under normal climatic conditions, on monuments, the precipitation of calcium sulfate from aqueous solution will therefore be predominantly gypsum. Anhydrite forms when the temperature in solution is higher than 40°-60°C. However, in parallel with this reaction, metastable hemihydrate can also precipitate being subsequently transformed into the more stable dihydrate form. <br> | |||
When heating the dihydrate (as a solid and dry) to approximately 50°C, the chemically combined water is lost leaving the hemihydrate. However, a complete transition to hemihydrate only takes place at temperatures of around 100°C. If the dihydrate is heated to 500-600°C, the anhydrous calcium sulfate is formed. At temperatures above 1000°C the thermal decomposition into calcium oxide and SO<sub>3</sub> is effected. | |||
<br clear="all"> | |||
=== Hygroscopicity === | |||
[[ | [[File:Veraenderung Loeslichkeit durch Fremdionen Gips .JPG|thumb|right|350px|'''Figure 3''': | ||
The modification of the solubility of gypsum in water, in the presence of [[halite]], is shown in Figure 3. If the concentration of halite is approximately 140 g/l in aqueous solution, around 8 g gypsum is dissolved (Angaben nach <bib id="DAns:1933"/>)]]The pure gypsum salt has no defined [[deliquescence|deliquescence]] point. If, in the presence of halite, the relative humidity levels exceed 90% RH, gypsum crystals may dissolve, due to the deliqusecent behavior of halite. A decrease of humidity levels to approximately 75% RH will result in the recrystallization of gypsum. | |||
=== | === Crystallization pressure === | ||
At crystallization in aqueous solution, with the saturation at a ration of 2:1, gypsum produces a linear growth pressure of 28,2-33,4 N/mm<sup>2</sup> within a temperature range of 0-50°C. In comparison with other damaging salts, these values lie in the middle range of a calculated scale of values reaching from 7,2 to 65,4 N/mm<sup>2</sup> [according to <bib id="Winkler:1975"/>]. | |||
=== | === Hydration pressure === | ||
Gypsum as a constituent of an object, can only release the water of crystallization (chemically combined water) at temperatures of approx. 50°C, i.e. it will normally not dehydrate. In contrast the enclosure of the water of crystallization is possible, if anhydrite or hemihydrate are present in a monument. Both processes are associated with a change in volume (of 31.9 % at the conversion from hemihydrate- gypsum) and the emergence of [[Schadensmechanismen|hydration pressure]] [values according to <bib id="Sperling.etal:1980"/>]. In the instance of a conversion from hemihydrate- gypsum (keyword Gipstreiben) at temperatures ranging from 0-20°C and an RH or 80%, a hydration pressure of 114 –160 N/mm<sup>2</sup> can result -an extremely high value [according to <bib id="Stark.etal:1996"/>]. | |||
== | == Conversion reaction == | ||
The hazardous character of gypsum to historic substance, is connected to the conversion reaction [[calcite]]- gypsum. The gypsum molecules formed by calcite hold a volume, that exceeds the volume of the original calcite molecule by about 100%. In this context a relevant damage factor is the modification of the water solubility. Calcite has a water solubility of approx. 0,014g/l (20°C) and is therefore more difficult to dissolve than gypsum. When a conversion to gypsum takes place the result is a more water sensitive system. N.B. The research by Snethlage and Wendler <bib id="Snethlage.etal:1998"/> analyses the influence of gypsum on the linear hygroscopic expansion of certain sandstone materials. The damages and the change in swelling behavior of the material was explained through the influence of gypsum. | |||
<br> | <br> | ||
== | == Analytical identification == | ||
<!-- | <!-- | ||
=== | === Micro-chemistry === | ||
--> | --> | ||
=== Microscopy === | |||
=== | |||
''' | '''Laboratory examination:''' Gypsum is slightly water soluble, therefore gypsum-containing sample material only dissolves slightly, when mixed with distilled Water. In solution, gypsum- containing sample material recrystallizes by carefully concentrating the solvent. At first, single needles form, then increasingly needle- like gypsum aggregate in proximity of the seam of the solvent emerges. Alternatively, sample material can be dissolved in hydrochloric acid, which also leads to the formation of crystal needles. Compared to other salts that can recrystallize in needle-like shapes, e.g. [[natrite|sodium carbonate]], gypsum needles are clearly shorter.<br> | ||
''' | '''Refraction indices:''' | ||
n<sub>x</sub> = 1.521; n<sub>y</sub> =1.523; n<sub>z</sub> =1.530<br> | |||
'''birefringence''': Δ = 0.009<br> | |||
'''crystal class''': monoclinic<br> | |||
'''[[Polarized light microscopy|Polarized light microscopy examination:]]'''<br> | |||
Apart from the typical acicular habit of gypsum crystals, (especially in recrystallized material) different morphological characteristics appear. These can be useful for identifying gypsum. Gypsum particles (in raw material samples) display shapes of rounded fragments and plate- like rhombohedra, clearly showing the inner cleavage planes. Furthermore, the occurrence of twinning shapes is typical for gypsum crystals, whether they are lath- shaped, tabular or lamellar. The assignment of refractive indices is carried out in accordance with the immersion method using media with indices nD=1,518 und nD=1,53. Due to the often small- scale particles the examination using the Schoeder van der Kolk method is more significant and reliable than the Becke- Line test. | |||
Gypsum crystals belong to the class of monoclinic crystals. Thus, they show, depending on the orientation of the single particle under the microscope, a parallel or respectively a symmetrical extinction, but mainly exhibit a characteristicly oblique axis position in the extinction position. On well developed crystal rhombi the oblique extinction can clearly be measured. Of all calcium sulfate crystals, gypsum has the lowest birefringence. Under crossed polarizers, gypsum has very low interference colors, lying within the gray to yellowish white range of the first order, (of course depending on the thickness of the particles). | |||
''' | <br> | ||
'''Possibility for mistakes:'''<br> | |||
The Analysis methods mentioned above clearly identify gypsum, provided the following evaluation criteria are explicitly clarified.<br> | |||
< | *low water solubility | ||
*characteristic needle- like morphology of the recrystallized particles | |||
*all observable indices have a n<sub>D</sub> –value from 1,518 and 1,530 | |||
*gypsum crystal show low interference colors | |||
*gypsum crystals have an oblique extinction | |||
{|border="2" cellspacing="0" cellpadding="4" width="60%" align="left" class="wikitable" | |||
{|border="2" cellspacing="0" cellpadding="4" width=" | |+''Table 1: Salt phases with a gypsum- like chemical and optical properties'' | ||
|+'' | |||
|- | |- | ||
|bgcolor = "#F0F0F0"|''' | |bgcolor = "#F0F0F0"|'''salt phase''' | ||
|bgcolor = "#F0F0F0"|''' | |bgcolor = "#F0F0F0"|'''differentiating features''' | ||
|- | |- | ||
|bgcolor = "#F7F7F7"|'''[[ | |bgcolor = "#F7F7F7"|'''[[Syngenite]]''' K<sub>2</sub>Ca(SO<sub>4)</sub> • 2H<sub>2</sub>O | ||
|bgcolor = "#FFFFEO"| | |bgcolor = "#FFFFEO"|all observable indices; 1,518 | ||
|- | |- | ||
|bgcolor = "#F7F7F7"|'''[[ | |bgcolor = "#F7F7F7"|'''[[Tachyhydrite]]''' CaMg<sub>2</sub>Cl<sub>6</sub> • 12H<sub>2</sub>O | ||
|bgcolor = "#FFFFEO"| | |bgcolor = "#FFFFEO"|mostly observable index < 1,518 / only parallel and symmetrical extinction | ||
|- | |- | ||
|bgcolor = "#F7F7F7"|'''[[ | |bgcolor = "#F7F7F7"|'''[[Hydromagnesite]]''' Mg<sub>5</sub>[OH(CO<sub>3</sub>)<sub>2</sub>]<sub>2</sub> • 4H<sub>2</sub>O | ||
|bgcolor = "#FFFFEO"| | |bgcolor = "#FFFFEO"|mostly one index > 1,53 | ||
|} | |} | ||
<br> | <br clear="all"> <br> | ||
<!-- | <!-- | ||
== | == X- Ray diffraction == | ||
== Raman- | == Raman-spectroscopy == | ||
== DTA / TG == | == DTA / TG == | ||
== IR- | == IR-spectroscopie == | ||
= | == Handling of damage caused by gypsum == | ||
--> | --> | ||
| Line 168: | Line 166: | ||
<br clear=all> | <br clear=all> | ||
=== Under the polarising microscope === | === Under the polarising microscope === | ||
| Line 183: | Line 180: | ||
Image:CaSO4 pol 400x 01.JPG|Calcium sulfate crystallized out of a solution in water on a glass slide | Image:CaSO4 pol 400x 01.JPG|Calcium sulfate crystallized out of a solution in water on a glass slide | ||
Image:CaSO4+NaCl reale Probe 01.JPG|Calcium sulfate with sodium chloride of a real sample, Gypsum crystallized out of a solution in water on a glass slide | Image:CaSO4+NaCl reale Probe 01.JPG|Calcium sulfate with sodium chloride of a real sample, Gypsum crystallized out of a solution in water on a glass slide | ||
Image:CaSO4+NaCl reale Probe 02.JPG|Calcium sulfate with sodium chloride of a real sample, Gypsum crystallized out of a solution in water on a glass slide | Image:CaSO4+NaCl reale Probe 02.JPG|Calcium sulfate with sodium chloride of a real sample, Gypsum crystallized out of a solution in water on a glass slide | ||
Image:HJS CaSO4 092503-1.jpg|Calcium sulfate, Gypsum crystallized out of a solution in water on a glass slide | Image:HJS CaSO4 092503-1.jpg|Calcium sulfate, Gypsum crystallized out of a solution in water on a glass slide | ||
</gallery> | |||
</gallery> | <br clear=all> | ||
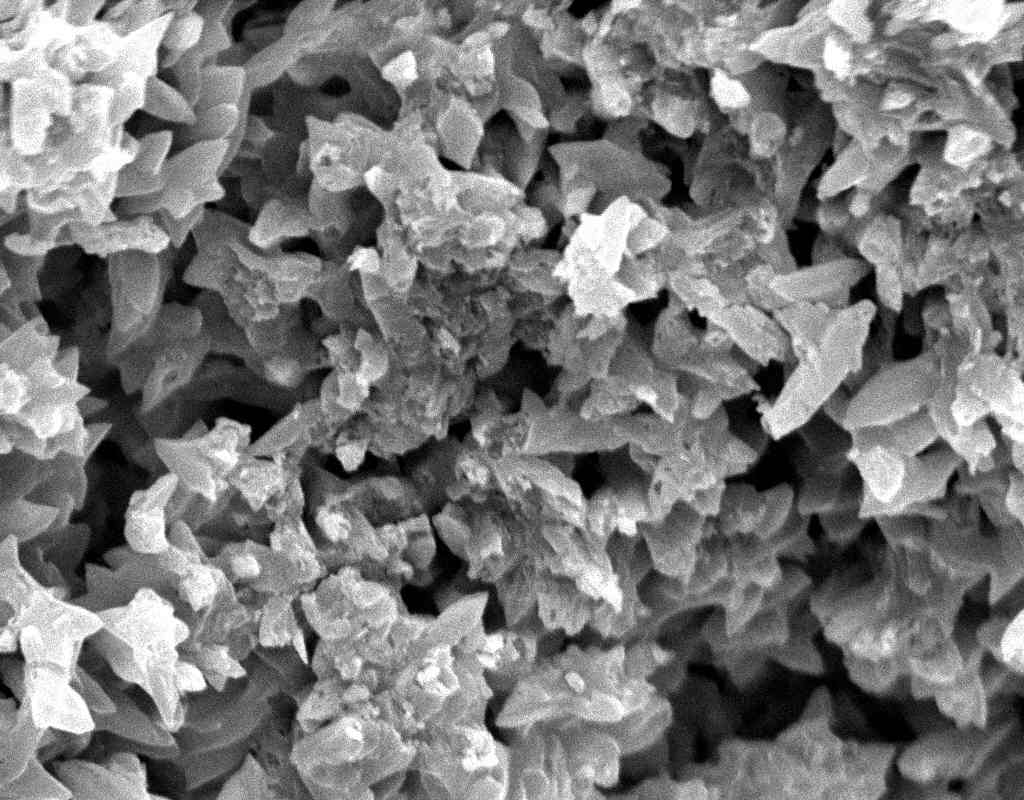
== Under the Scanning Electron Microscope (SEM)== | |||
<gallery caption="In a SEM" widths="200px" heights="150px" perrow="3"> | |||
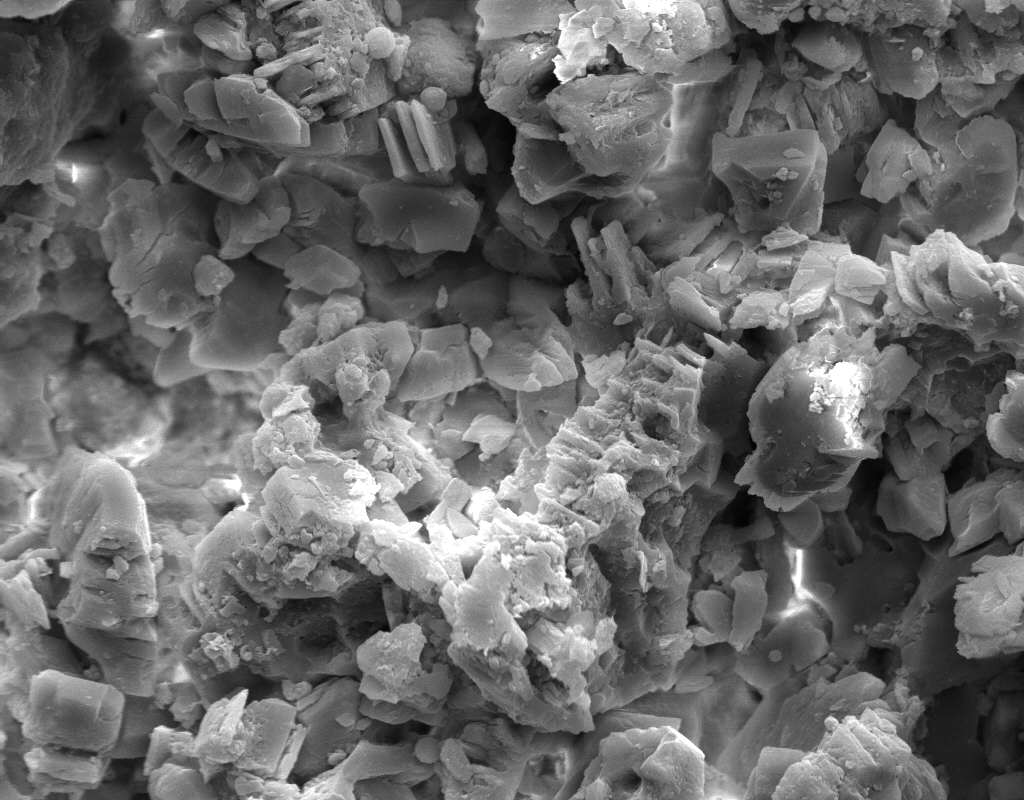
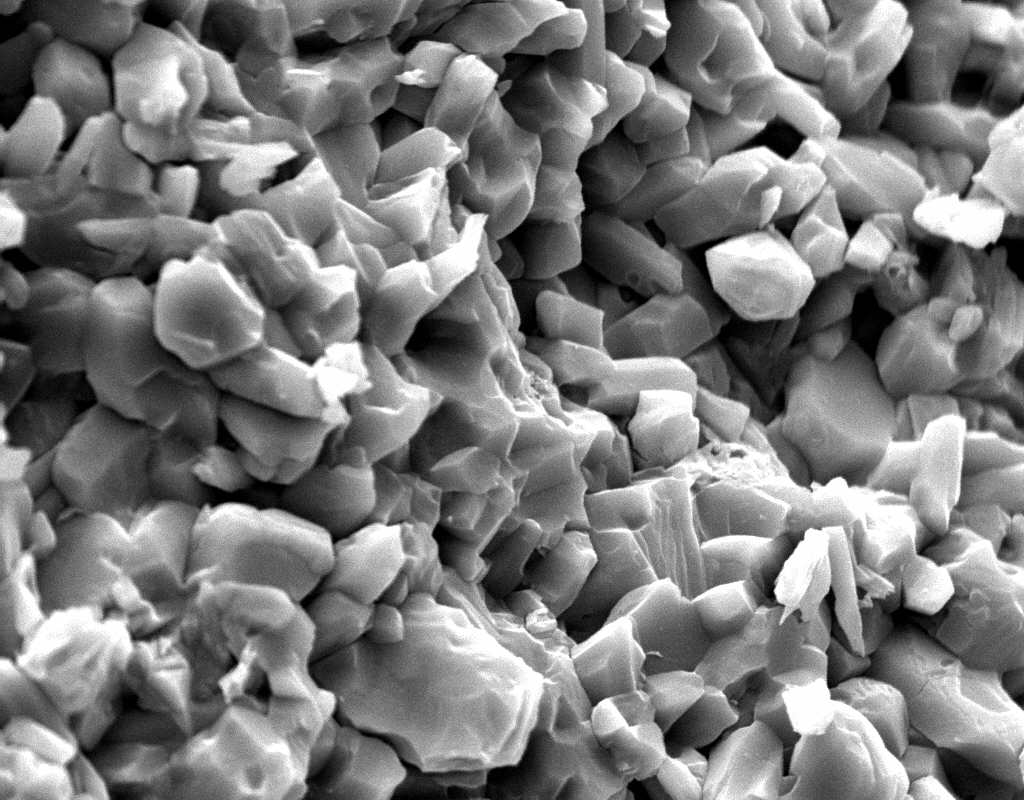
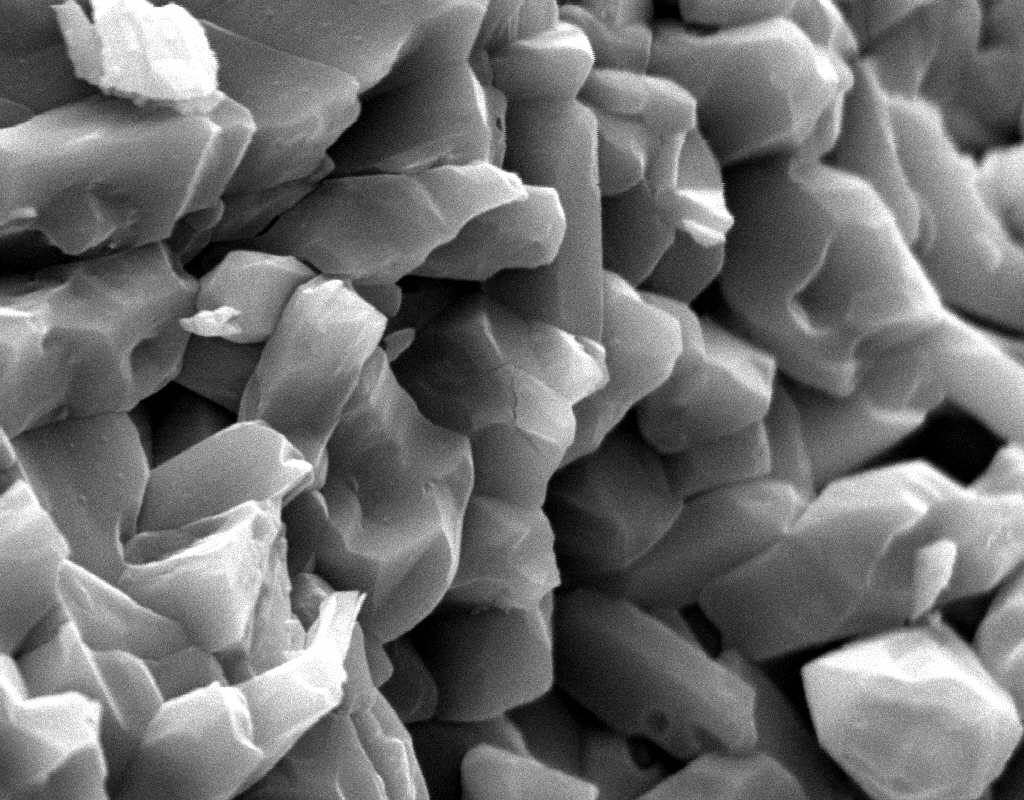
Image:SA100_1.jpg | SEM micrograph of gypsum crystals. | |||
Image:SG2-2.jpeg | SEM of gypsum crystals. | |||
Image:SG2-3.jpg | SEM of gypsum crystals. | |||
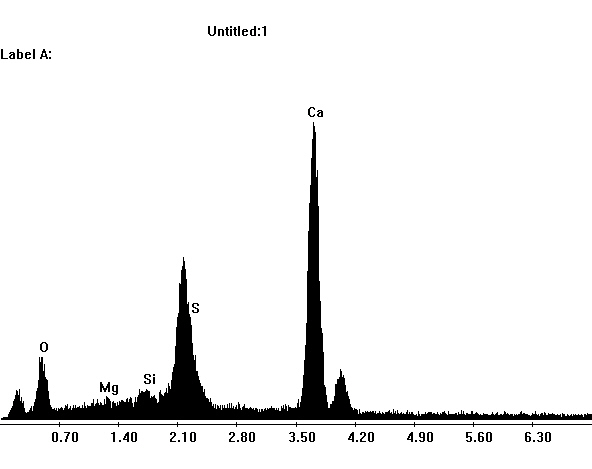
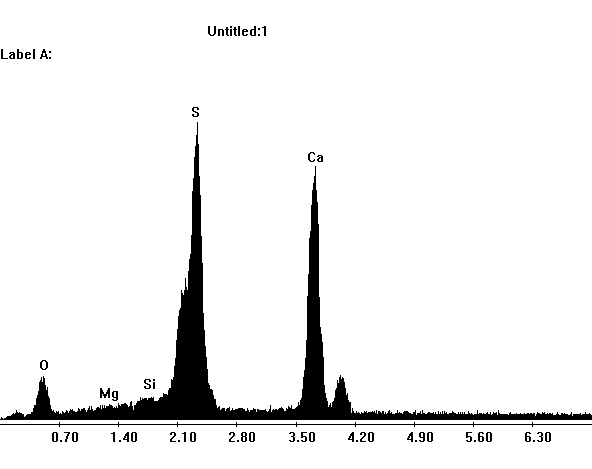
Image:SG3-SPC2.jpeg|EDX spectra of gypsum crystals. | |||
Image:SG3-3.jpeg | SEM of gypsum crystals. | |||
Image:SG3-4.jpeg | SEM of gypsum crystals. | |||
Image:SG1-5.jpeg | SEM of gypsum crystals. | |||
Image:SG1-SPC.jpeg| EDX spectra of gypsum crystals. | |||
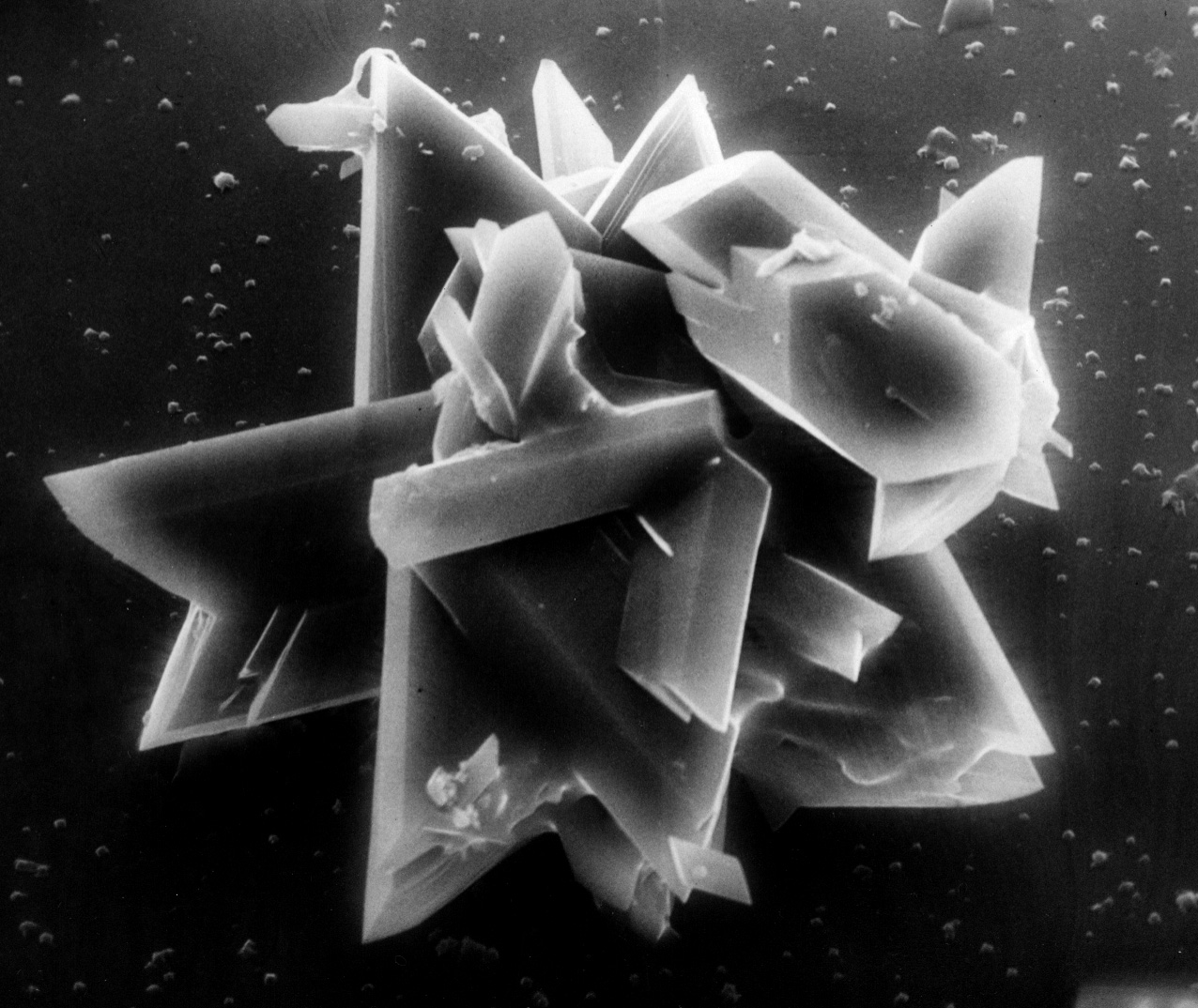
Image:Gypsum star.jpg|SEM of gypsum crystals grown from the hemihydrate.</gallery> | |||
<br clear=all> | <br clear=all> | ||
< | == Weblinks == | ||
<references /> | |||
== Literature == | |||
<biblist/> | |||
< | More Literature : | ||
<bibprint filter="year:2011, author:%Badosa%"/> | |||
<bibprint filter="year:2007, volume:52, author:%Charola%"/> | |||
<bibprint filter="year:1901, volume:5, author:%Cameron%"/> | |||
<bibprint filter="year:1989, volume:34, author:%Ahad%"/> | |||
<bibprint filter="year:1991, author:%Livingston%"/> | |||
<bibprint filter="year:1997, author:%Neumann%, author:%Juling%"/> | |||
<bibprint filter="year:1994, author:%Schlütter%"/> | |||
<bibprint filter="year:2009, author:%Zehnder%"/> | |||
[[Category:Gipsum]][[Category:Sulphate]][[Category:Salt]][[Category: | |||
[[Category:Gipsum]][[Category:Sulphate]][[Category:Salt]][[Category:editing]][[Category:Sulfate]][[Category:Müller,Tim]][[Category:Mainusch,Nils]][[Category:Schwarz,Hans-Jürgen]][[Category:List]] | |||
Latest revision as of 10:55, 3 May 2023
Authors: Hans-Jürgen Schwarz , Nils Mainusch, Tim Müller
English Translation by Sandra Leithäuser
back to Sulfate
| Gypsum[1][2] | |

| |
| Mineralogical name | Gypsum |
| Chemical name | Calcium sulfate dihydrate |
| Trivial name | Alabaster, Satin Spar, Selenite, |
| Chemical formula | CaSO4•2H2O |
| Other forms | CaSO4 (Anhydrite) CaSO4•0.5H2O (Bassanite) |
| Crystal system | monoclinic |
| Crystal structure | |
| Deliquescence humidity 20°C | > 99% RH at 20°C |
| Solubility (g/l) at 20°C | 2.14 g/l |
| Density (g/cm³) | 2.31 g/cm³ |
| Molar volume | 74.69 cm3/mol |
| Molar weight | 172.17g /mol |
| Transparency | transparent to opaque |
| Cleavage | perfect, clearly visible formation of fibre |
| Crystal habit | flat, prismatic, needle- like crystal, granular, massive aggregate |
| Twinning | very common |
| Phase transition | |
| Chemical behavior | hardly soluble in water |
| Comments | |
| Crystal Optics | |
| Refractive Indices | α = 1.5207 β = 1.5230 γ = 1.5299 |
| Birefringence | Δ = 0.0092 |
| Optical Orientation | biaxial positive |
| Pleochroism | colorless |
| Dispersion | 58° |
| Used Literature | |
| [Robie.etal:1978]Title: Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar pressure and higher temperatures Author: Robie R.A., Hemingway B.S.; Fisher J.A.  [Dana:1951]Title: Dana's System of Mineralogy [Dana:1951]Title: Dana's System of MineralogyAuthor: Dana J.D. 
| |
Calcium sulfate and gypsum[edit]
Abstract[edit]
The article discusses the system CaSO4-H2O, in reference to gypsum. Gypsum is one of the most important salts in the deterioration of building materials and, particularly, wall-paintings. Objects exposed to exterior conditions where air pollution is present are the most prone to damage from gypsum. The appearance and mechanism of the damage, as well as the examination methods are described. Images, microphotographs and examples from practical experiences illustrate the subject.
Introduction[edit]
Gypsum is one of the most common salts causing deterioration of inorganic porous building materials. It is present on most exterior exposed surfaces and even in interior environments, under different shapes and inducing various deterioration patterns.
Gypsum, one of the most prevalent minerals, forms by precipitation from aqueous solution at temperatures under approximately 40°C. When the solution reaches higher temperatures (> 60°C) anhydrite precipitates out. Calcium sulfate and calcium sulfate dihydrate are often present in rocks. Hemihydrate does not occur naturally.
Gypsum occurs naturally in salt deposits and deserts, where "desert rose" crystals form in combination with quartz inclusions. In natural salt deposits, gypsum and anhydrite sometimes form a caprock, i.e., a massive layer of material covering the deposit. Synthetic gypsum is produced in coal-fired power plants, as a by-product of flue-gas desulfurization.
Origin and formation of gypsum on monuments[edit]
On monuments made from porous inorganic building materials, particularly in urban environment where anthropogenic air pollutants are present, such as sulfur oxides (SOx) that eventually convert to sulfuric acid in the presence of moisture, the reaction of these gases with any calcium carbonate present in limestone, sandstone, mortars, renders, results in the formation of gypsum. The reaction taking place can be simplified as follows:
CaCO3 + H2SO4 → CaSO4 + H2O + CO2
It is to be remembered that gypsum can be a major component of some mortars, plasters and even some building stones (e.g., selenite, used for the base of the Garrisenda Tower in Bologna, Italy) thus being an integral part of the monuments fabric.
Damage potential and weathering activity[edit]
Solubility properties[edit]
Gypsum belongs to the group of salts with a low solubility in aqueous solution and therefore is less mobile than the more soluble ones. However, when other ions are present, its solubility can be significantly increased. For example, when halite, NaCl, is present, the solubility of gypsum can be increased by a factor four depending on the concentration ratio of the two salts.
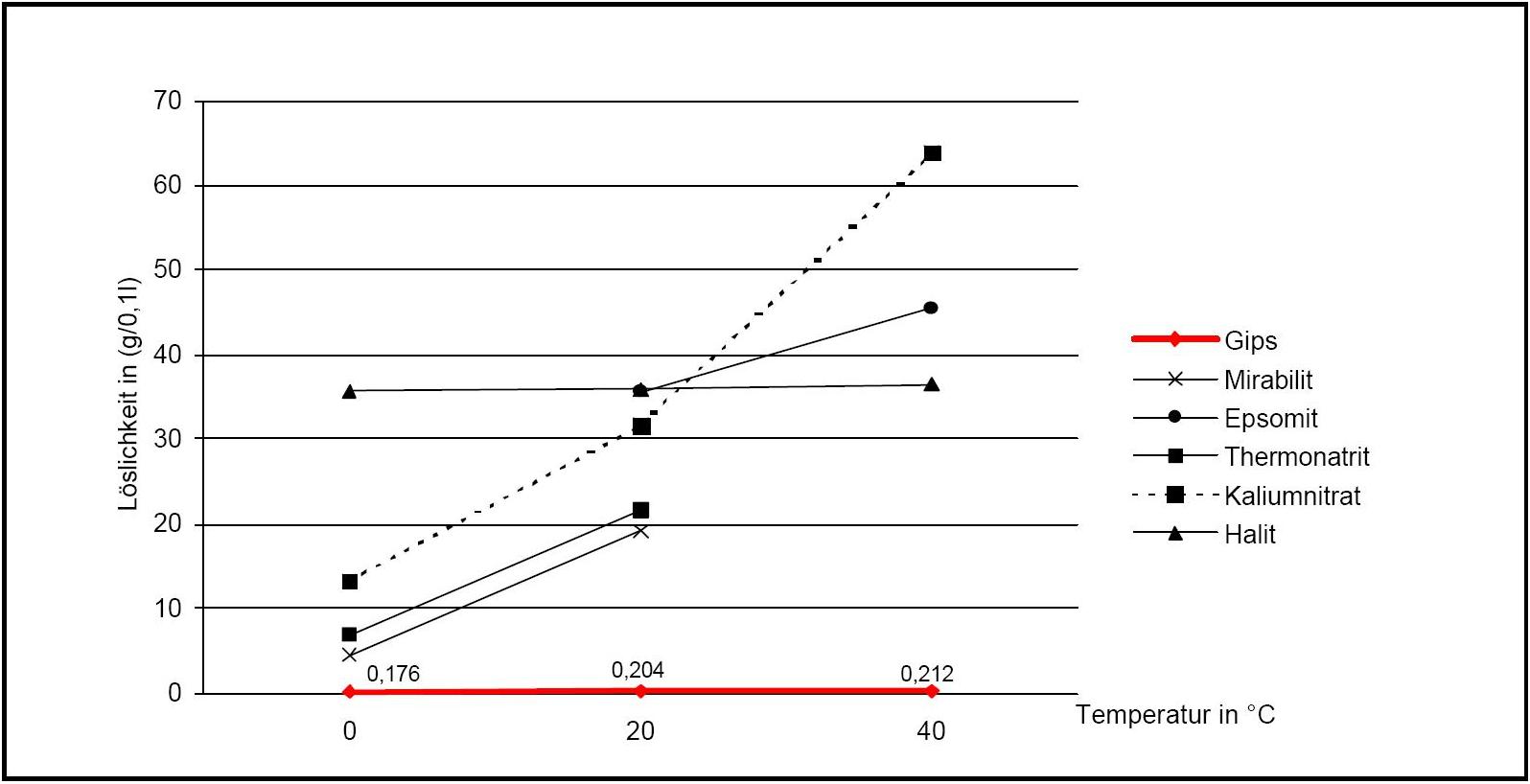
Author: Stark, Jochen; Stürmer, Sylvia
 )
)Hydration behavior[edit]
The system CaSO4 –H2O:
Calcium sulfate can appear in three different hydrate phases:
- Anhydrite (CaSO4)- the above mentioned anhydrous form.
- Bassanite (hemihydrate) (CaSO4•0.5H2O)- a metastable form.
- Gypsum (CaSO4•2H2O)- calcium sulfate dihydrate.
Anhydrite exists in different varieties with different chemical properties, such as different solubilities in water, depending on the conditions of its formation. The same applies to bassanite, the hemihydrate.
The transition temperature in aqueous solution for the gypsum-bassanite (dihydrate to hemihydrate) is in the 40°C-66°C range. Under normal climatic conditions, on monuments, the precipitation of calcium sulfate from aqueous solution will therefore be predominantly gypsum. Anhydrite forms when the temperature in solution is higher than 40°-60°C. However, in parallel with this reaction, metastable hemihydrate can also precipitate being subsequently transformed into the more stable dihydrate form.
When heating the dihydrate (as a solid and dry) to approximately 50°C, the chemically combined water is lost leaving the hemihydrate. However, a complete transition to hemihydrate only takes place at temperatures of around 100°C. If the dihydrate is heated to 500-600°C, the anhydrous calcium sulfate is formed. At temperatures above 1000°C the thermal decomposition into calcium oxide and SO3 is effected.
Hygroscopicity[edit]
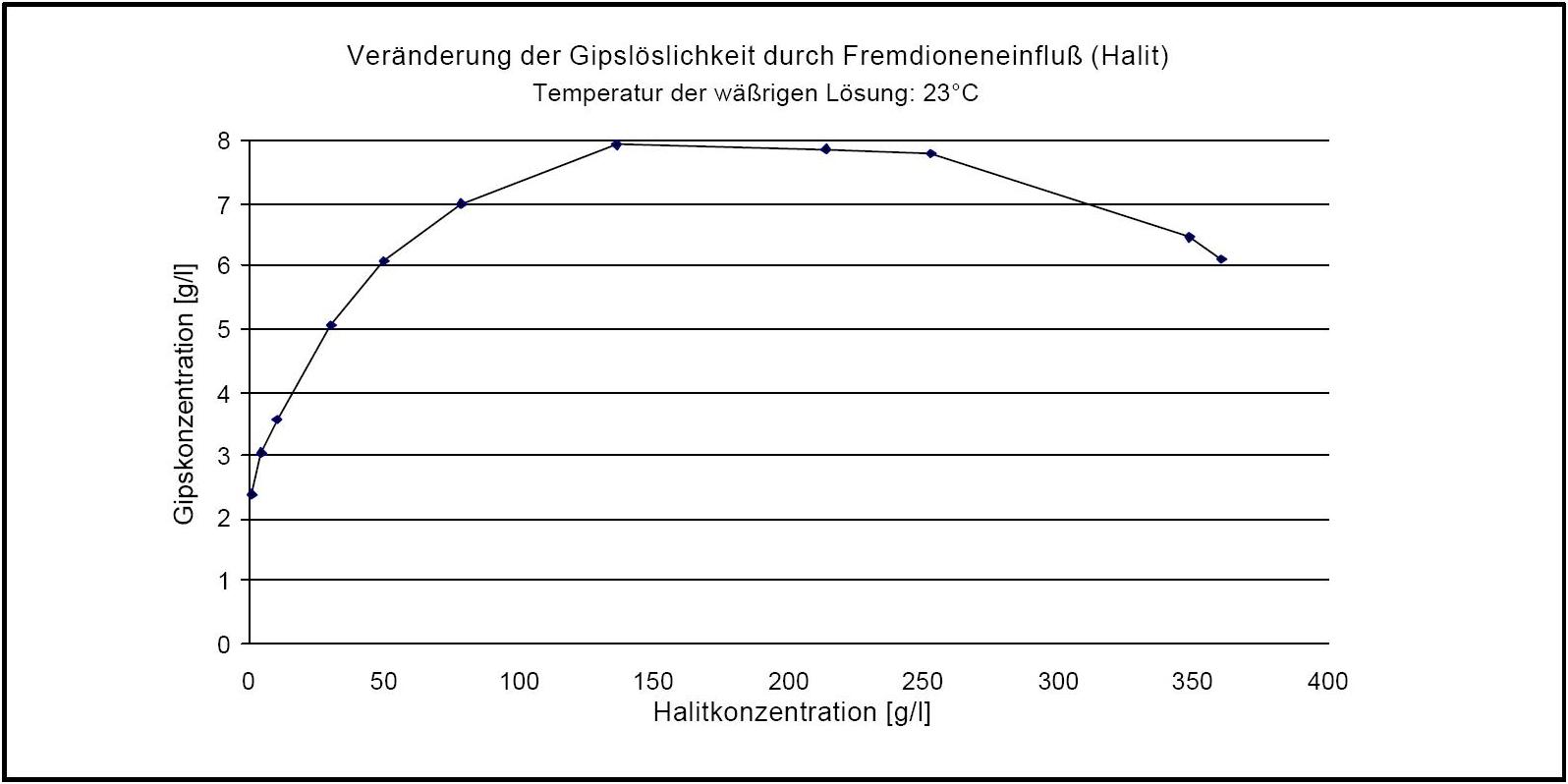
Author: d'Ans, J.
 )
)The pure gypsum salt has no defined deliquescence point. If, in the presence of halite, the relative humidity levels exceed 90% RH, gypsum crystals may dissolve, due to the deliqusecent behavior of halite. A decrease of humidity levels to approximately 75% RH will result in the recrystallization of gypsum.
Crystallization pressure[edit]
At crystallization in aqueous solution, with the saturation at a ration of 2:1, gypsum produces a linear growth pressure of 28,2-33,4 N/mm2 within a temperature range of 0-50°C. In comparison with other damaging salts, these values lie in the middle range of a calculated scale of values reaching from 7,2 to 65,4 N/mm2 [according to [Winkler:1975]Title: Stone: Properties, Durability in Man´s Environment
Author: Winkler, Erhard M. ].
].
Hydration pressure[edit]
Gypsum as a constituent of an object, can only release the water of crystallization (chemically combined water) at temperatures of approx. 50°C, i.e. it will normally not dehydrate. In contrast the enclosure of the water of crystallization is possible, if anhydrite or hemihydrate are present in a monument. Both processes are associated with a change in volume (of 31.9 % at the conversion from hemihydrate- gypsum) and the emergence of hydration pressure [values according to [Sperling.etal:1980]Title: Salt Weathering on Arid Environment, I. Theoretical ConsiderationsII. Laboratory Studies
Author: Sperling, C.H.B.and Cooke, R.U. ]. In the instance of a conversion from hemihydrate- gypsum (keyword Gipstreiben) at temperatures ranging from 0-20°C and an RH or 80%, a hydration pressure of 114 –160 N/mm2 can result -an extremely high value [according to [Stark.etal:1996]Title: Bauschädliche Salze
]. In the instance of a conversion from hemihydrate- gypsum (keyword Gipstreiben) at temperatures ranging from 0-20°C and an RH or 80%, a hydration pressure of 114 –160 N/mm2 can result -an extremely high value [according to [Stark.etal:1996]Title: Bauschädliche Salze
Author: Stark, Jochen; Stürmer, Sylvia ].
].
Conversion reaction[edit]
The hazardous character of gypsum to historic substance, is connected to the conversion reaction calcite- gypsum. The gypsum molecules formed by calcite hold a volume, that exceeds the volume of the original calcite molecule by about 100%. In this context a relevant damage factor is the modification of the water solubility. Calcite has a water solubility of approx. 0,014g/l (20°C) and is therefore more difficult to dissolve than gypsum. When a conversion to gypsum takes place the result is a more water sensitive system. N.B. The research by Snethlage and Wendler [Snethlage.etal:1998]Title: Steinzerfall und Steinkonservierung - neueste Ergebnisse der Münchner Forschungen
Author: Snethlage, Rolf; Wendler, Eberhard analyses the influence of gypsum on the linear hygroscopic expansion of certain sandstone materials. The damages and the change in swelling behavior of the material was explained through the influence of gypsum.
analyses the influence of gypsum on the linear hygroscopic expansion of certain sandstone materials. The damages and the change in swelling behavior of the material was explained through the influence of gypsum.
Analytical identification[edit]
Microscopy[edit]
Laboratory examination: Gypsum is slightly water soluble, therefore gypsum-containing sample material only dissolves slightly, when mixed with distilled Water. In solution, gypsum- containing sample material recrystallizes by carefully concentrating the solvent. At first, single needles form, then increasingly needle- like gypsum aggregate in proximity of the seam of the solvent emerges. Alternatively, sample material can be dissolved in hydrochloric acid, which also leads to the formation of crystal needles. Compared to other salts that can recrystallize in needle-like shapes, e.g. sodium carbonate, gypsum needles are clearly shorter.
Refraction indices:
nx = 1.521; ny =1.523; nz =1.530
birefringence: Δ = 0.009
crystal class: monoclinic
Polarized light microscopy examination:
Apart from the typical acicular habit of gypsum crystals, (especially in recrystallized material) different morphological characteristics appear. These can be useful for identifying gypsum. Gypsum particles (in raw material samples) display shapes of rounded fragments and plate- like rhombohedra, clearly showing the inner cleavage planes. Furthermore, the occurrence of twinning shapes is typical for gypsum crystals, whether they are lath- shaped, tabular or lamellar. The assignment of refractive indices is carried out in accordance with the immersion method using media with indices nD=1,518 und nD=1,53. Due to the often small- scale particles the examination using the Schoeder van der Kolk method is more significant and reliable than the Becke- Line test.
Gypsum crystals belong to the class of monoclinic crystals. Thus, they show, depending on the orientation of the single particle under the microscope, a parallel or respectively a symmetrical extinction, but mainly exhibit a characteristicly oblique axis position in the extinction position. On well developed crystal rhombi the oblique extinction can clearly be measured. Of all calcium sulfate crystals, gypsum has the lowest birefringence. Under crossed polarizers, gypsum has very low interference colors, lying within the gray to yellowish white range of the first order, (of course depending on the thickness of the particles).
Possibility for mistakes:
The Analysis methods mentioned above clearly identify gypsum, provided the following evaluation criteria are explicitly clarified.
- low water solubility
- characteristic needle- like morphology of the recrystallized particles
- all observable indices have a nD –value from 1,518 and 1,530
- gypsum crystal show low interference colors
- gypsum crystals have an oblique extinction
| salt phase | differentiating features |
| Syngenite K2Ca(SO4) • 2H2O | all observable indices; 1,518 |
| Tachyhydrite CaMg2Cl6 • 12H2O | mostly observable index < 1,518 / only parallel and symmetrical extinction |
| Hydromagnesite Mg5[OH(CO3)2]2 • 4H2O | mostly one index > 1,53 |
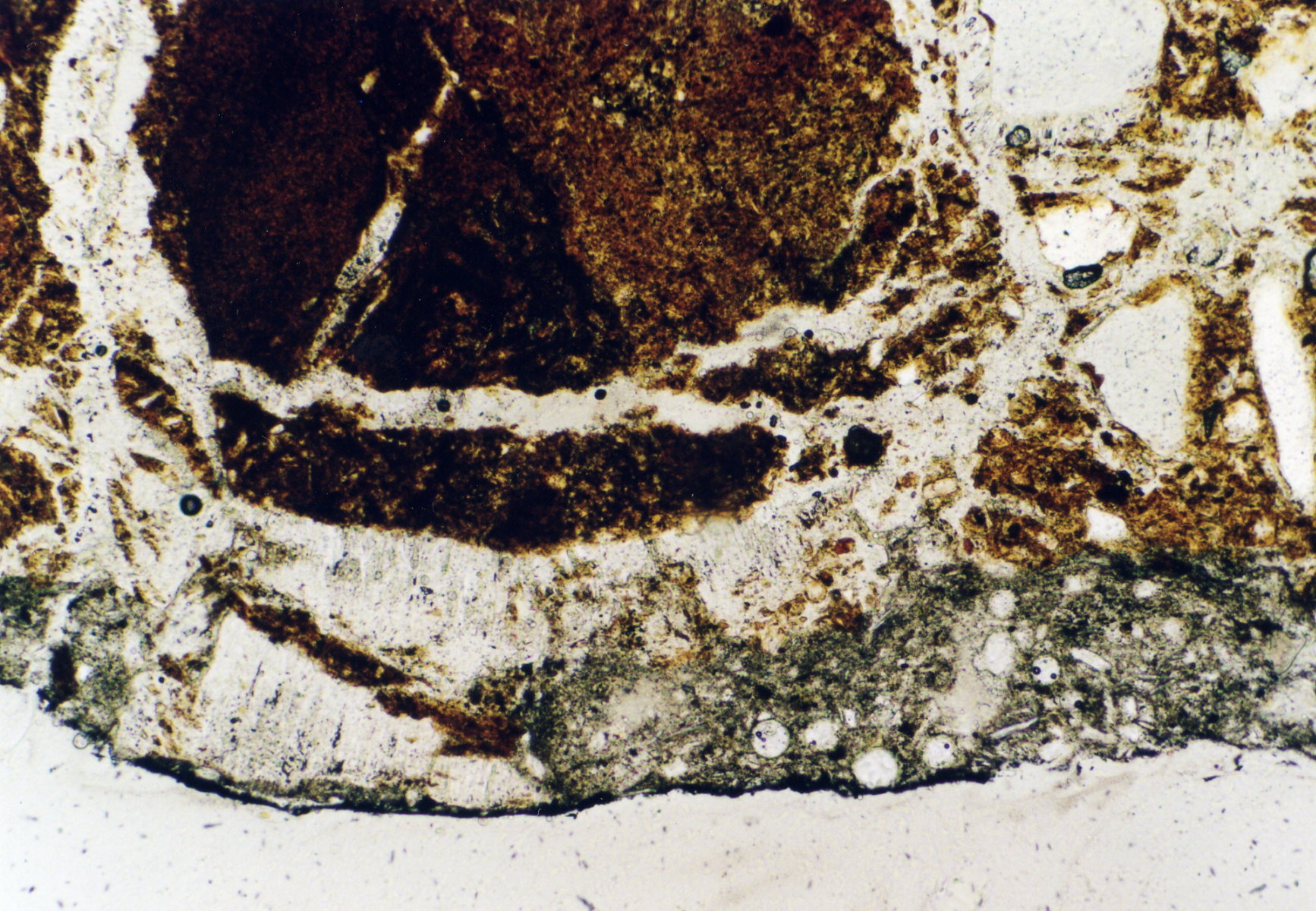
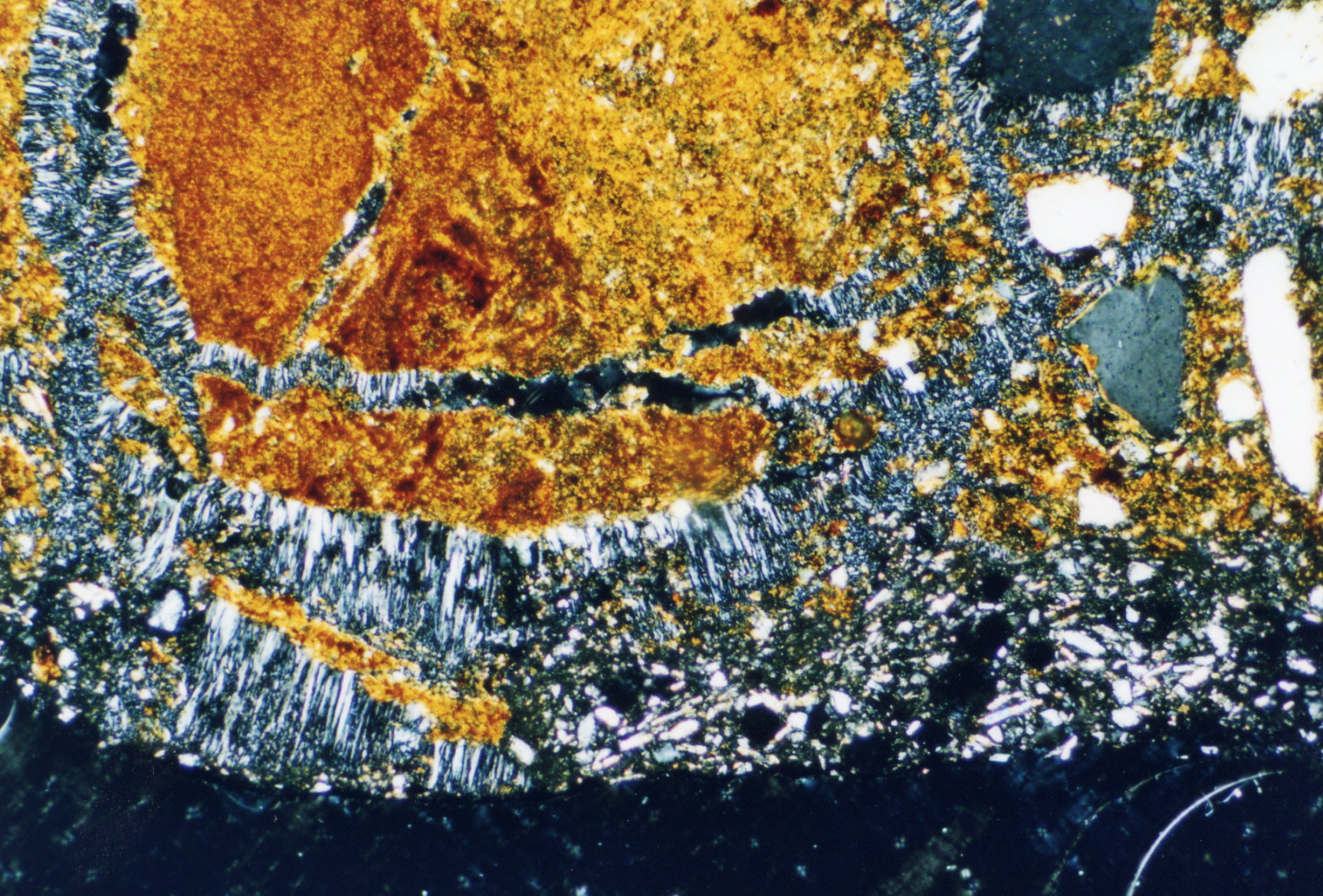
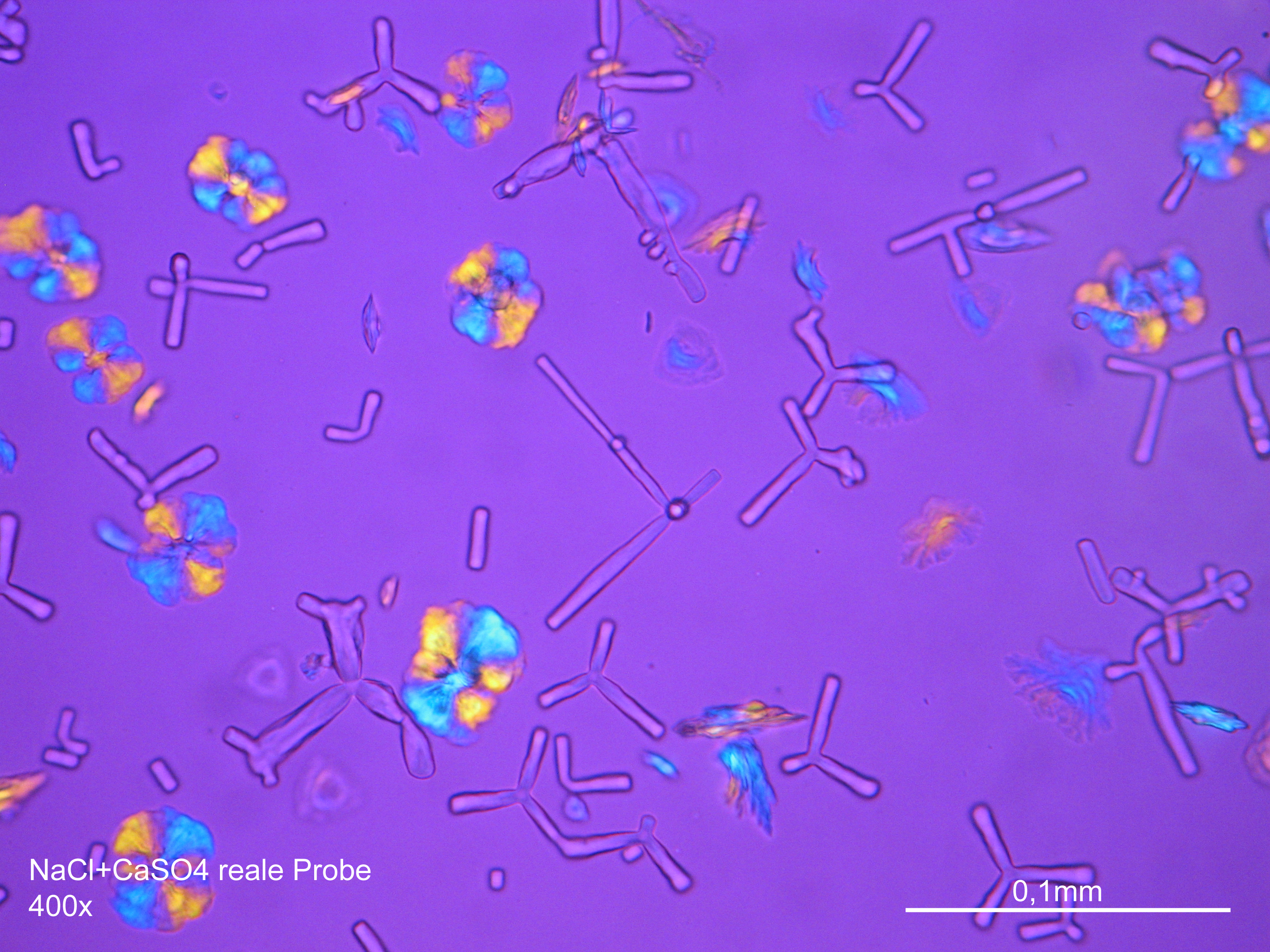
Photos of gypsum crystals and deterioration pattern caused by gypsum[edit]
On an object[edit]
Under the polarising microscope[edit]
- In thin section of bricks
- Gypsum crystallized out of a solution in water on a glass slide
Under the Scanning Electron Microscope (SEM)[edit]
- In a SEM
Weblinks[edit]
- ↑ http://webmineral.com/data/Gypsum.shtml seen on 30.07.2010
- ↑ http://www.mindat.org/min-1784.html seen on 30.07.2010
Literature[edit]
| [DAns:1933] | d'Ans, J. (1933): Die Lösungsgleichgewichte der Systeme der Salze ozeanischer Salzablagerungen, Verlagsgesellschaft für Ackerbau, M.B.H. Berlin |  |
| [Dana:1951] | Dana E.S. (eds.) Dana J.D. (1951): Dana's System of Mineralogy, 7, Wiley & Sons |  |
| [Robie.etal:1978] | Robie R.A., Hemingway B.S.; Fisher J.A. (1978): Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar pressure and higher temperatures. In: U.S. Geol. Surv. Bull, 1452 () |  |
| [Snethlage.etal:1998] | Snethlage, Rolf; Wendler, Eberhard (1998): Steinzerfall und Steinkonservierung - neueste Ergebnisse der Münchner Forschungen. In: Münchner Geologische Hefte, A 23, Festschrift zum 65. Geburtstag von Prof. Dr. Dietrich D. Klemm, (), 177-201 |  |
| [Sperling.etal:1980] | Sperling, C.H.B.and Cooke, R.U. (1980): Salt Weathering on Arid Environment, I. Theoretical ConsiderationsII. Laboratory Studies. In: Papers in Geography, 8 () |  |
| [Stark.etal:1996] | Stark, Jochen; Stürmer, Sylvia (1996): Bauschädliche Salze, Bauhaus-Univ. Weimar |  |
| [Winkler:1975] | Winkler, Erhard M. (1975): Stone: Properties, Durability in Man´s Environment, Springer Verlag, Wien |  |
More Literature :
| [Badosa.etal:2011] | Badosa, S.; Beck, K.; Brunetaud, X.; Al-Mukhtar, M. (2011): The role of gypsum in the phenomenon of spalling of stones. In: Ionannou, Ioannis; Theodoridou, Magdalini (eds.): Proceedings of the Conference "Salt Weathering on Buildings and Stone Sculptures", Limassol, Cyprus, 19.-22. Oct. 2011, 415. |   |
| [Charola.etal:2007] | Charola, A. Elena; Pühringer, Josef; Steiger, Michael (2007): Gypsum: a review of its role in the deterioration of building materials. In: Environmental Geology, 52 (2), 207-220, Url, 10.1007/s00254-006-0566-9 |  |
| [Cameron.etal:1901] | Cameron; Seidell (1901): Solubility of gypsum in aqeous solutions of certain electrolytes. In: Journal of Physical Chemistry, 5 (), 643-655 |  |
| [LalGauri.etal:1989] | Lal Gauri, K.; Chowdhury, Ahad N.; Kulshreshtha, Niraj P.; Punuru, Adinarayana R. (1989): The sulfation of marble and the treatment of gypsum crusts. In: Studies in Conservation, 34 (4), 201-6 |  |
| [Livingston:1991] | Livingston, R. (1991): The use of gypsum mortar in historic buildings. In: Brebbia, C. A.; Dominguez, J.; Escrig, F. (eds.): Structural Repair and Maintenance of Historic Buildings II, Computational Mechanics Publications, 157-165. |  |
| [Neumann.etal:1997] | Neumann, Hans-Hermann; Lork, A.; Steiger, Michael; Juling, Herbert (1997): Decay patterns of weathered quarz sandstones: Evidence of gypsum induced structural changes. In: Sveinsdottir, E.L. (eds.): Proceedings 6th Euroseminar on microscopy applied to building materials, Iceland Building Research Institute, 238-249. |  |
| [Schluetter.etal:1994] | Schlütter, Frank; Juling, Herbert; Blaschke, Rochus (1994): Black skins and gypsum crystallization on terra-cotta material--microscopical investigations on samples of the Schwerin Castle. In: Fitz, Stephen (eds.): NATO-CCMS pilot study "Conservation of historic brick structures:" proceedings of the 7th expert meeting, Venice, 22-24 November 1993, , 90-99. |  |
| [Zehnder.etal:2009] | Zehnder, K.; Schoch, O. (2009): Efflorescence of mirabilite, epsomite and gypsum traced by automated monitoring on-site. In: Journal of Cultural Heritage, 10 (3), 319-330, Url, 10.1016/j.culher.2008.10.009 |  |