LiCl Condensation Hygrometer: Difference between revisions
No edit summary |
|||
| Line 9: | Line 9: | ||
The LiCl condensation hygrometer '''(also called a DEWCON-sensor?? Habe ich nicht gefunden)''' is an electrolytic conductivity sensor. | The LiCl condensation hygrometer '''(also called a DEWCON-sensor?? Habe ich nicht gefunden)''' is an electrolytic conductivity sensor. | ||
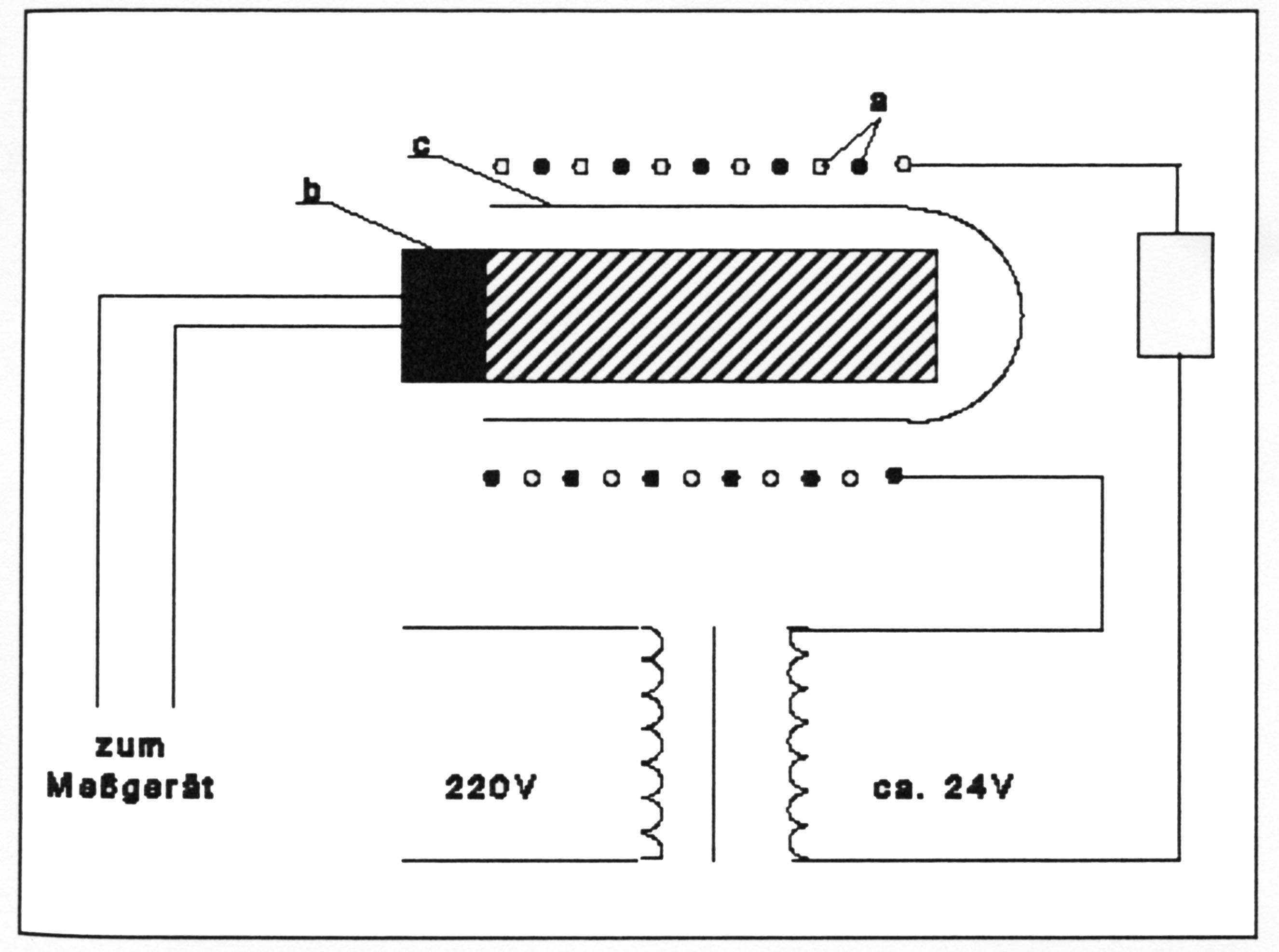
[[file:Schematischer Aufbau eines LiCL-Messelements.JPG|thumb| | [[file:Schematischer Aufbau eines LiCL-Messelements.JPG|thumb|right|300px|'''Figure 1''' - Schematischer Aufbau eines LiCl-Messelementes [Weber:1995]] | ||
The principle of operation is that the LiCl-salt containing ceramic sensor, which has been equipped with a heating meander and Pt 500 measuring resistor, heats up until it reaches the [[Deliquescence humidity|equilibrium moisture level/deliquescence humidity]] of the salt. At this point, no more electric current flows and the heating stops, resulting in a temperature drop. Now the sensor picks up moisture again and starts re-heating. After a while and at a specific temperature, the sensor adjusts to equilibrium. The temperature depends exclusively on the water vapor partial pressure of the ambient air. Automatically, and free of hysteresis, the water vapor pressure of the ambient air that is to be measured, is kept in equilibrium and the sensor temperature is a physically clear measure of the dew-point, similar to the automatic dew-point hygrometer. | The principle of operation is that the LiCl-salt containing ceramic sensor, which has been equipped with a heating meander and Pt 500 measuring resistor, heats up until it reaches the [[Deliquescence humidity|equilibrium moisture level/deliquescence humidity]] of the salt. At this point, no more electric current flows and the heating stops, resulting in a temperature drop. Now the sensor picks up moisture again and starts re-heating. After a while and at a specific temperature, the sensor adjusts to equilibrium. The temperature depends exclusively on the water vapor partial pressure of the ambient air. Automatically, and free of hysteresis, the water vapor pressure of the ambient air that is to be measured, is kept in equilibrium and the sensor temperature is a physically clear measure of the dew-point, similar to the automatic dew-point hygrometer. | ||
Revision as of 11:05, 28 October 2012
Author: Hans-Jürgen Schwarz
English Translation by Sandra Leithäuser
back to Moisture measurement methods
Abridged Version
The LiCl condensation hygrometer (also called a DEWCON-sensor?? Habe ich nicht gefunden) is an electrolytic conductivity sensor.
The principle of operation is that the LiCl-salt containing ceramic sensor, which has been equipped with a heating meander and Pt 500 measuring resistor, heats up until it reaches the equilibrium moisture level/deliquescence humidity of the salt. At this point, no more electric current flows and the heating stops, resulting in a temperature drop. Now the sensor picks up moisture again and starts re-heating. After a while and at a specific temperature, the sensor adjusts to equilibrium. The temperature depends exclusively on the water vapor partial pressure of the ambient air. Automatically, and free of hysteresis, the water vapor pressure of the ambient air that is to be measured, is kept in equilibrium and the sensor temperature is a physically clear measure of the dew-point, similar to the automatic dew-point hygrometer.
LiCl-solutions form different hydrates, depending on the temperature. The sensor may become unstable at the transition temperature of the salts, because the chemically bound water content of the undissolved salt changes abruptly, which is associated with an enthalpy change.
Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle LiCl \cdot 3 H_2O \rightarrow LiCl \cdot 2 H_2O (- 20 ^\circ C)}
Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle LiCl \cdot 2 H_2O \rightarrow LiCl \cdot H_2O (20 ^\circ C)}
Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle LiCl \cdot H_2O \rightarrow LiCl (100 ^\circ C)}
The sensor is to be protected from liquid water. Contamination of the salt may lead to drifts. (Ist mir nicht ganz klar wie das gemeint ist – z.B. Salt contamination of the sensor /LiCl salt/liquid water?) Der Fühler ist vor flüssigem Wasser zu schützen. Eine Drift kann durch Verunreinigungen des Salzes ausgehen. It is important to always run the LiCl covered sensor with heater voltage, because otherwise it takes up water hygroscopically and leaves the prescribed measuring position. Then, the unit is no longer operational. The lower the absolute humidity, the longer it takes for the sensor to respond to a signal.