Microscopic identification of salts: Difference between revisions
| (11 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
Author: [[user:Hschwarz|Hans-Jürgen Schwarz]] | Author: [[user:Hschwarz|Hans-Jürgen Schwarz]] | ||
<br> | <br> | ||
English Translation by [[user:SLeithaeuser|Sandra Leithäuser]]<br> | |||
<br> | <br> | ||
back to [[Polarized light microscopy]] or [[Analysis of Salts]] | back to [[Polarized light microscopy]] or [[Analysis of Salts]] | ||
| Line 8: | Line 9: | ||
== Abstract == | == Abstract == | ||
This section describes the | This section describes the identification of salts using [[polarized light microscopy]]. | ||
== Method == | |||
The examination of salts can be carried out using several different methods, such as microscopy, spot test analysis, X-ray diffraction. It is important to determine both the anions and cations in the salt(s) and, if possible, the specific phases of the actual salts. In general, laboratories carrying out routine chemical analysis do not usually determine the presence of carbonate ions, and therefore this salt is not identified, although they are often present and can be responsible for the observed efflorescence and eventual deterioration. | |||
Two different approaches to microscopic analysis of salts are presented here: | |||
The first approach considers mainly the analysis of the salts, that is, the salt crystals actually taken from the object. | |||
The | The second approach analyses the recrystallized salts obtained from the aqueous extraction of either the salts themselves or of a sample of the salt-contaminated material. Both possibilities lead to salt crystallizations that vary, depending on the different kind of salt, as described below. See also [[Micro-chemical testing]]. | ||
== Determination of | == Determination of individual salts in aqueous extract == | ||
Before the actual determination of salts, the sample to be examined (pure salt or material/salt mixture) is mixed with a few drops of distilled water, producing an aqueous [[Micro-chemical testing#Test methods|extract as discussed by Bläuer]]. A few drops of this extract are placed onto a microscope slide and observed under the polarizing microscope. It is important to observe the crystallization of the salts from the beginning, i.e., from the appearance of the first small crystals until the end of the crystallization process. Only then, the right conclusions can be drawn, because salts that crystallize later may cover up some salts completely, and therefore their crystal shape may become difficult to recognize and to identify. The continuous documentation of the process is therefore an important option. | Before the actual determination of salts, the sample to be examined (pure salt or material/salt mixture) is mixed with a few drops of distilled water, producing an aqueous [[Micro-chemical testing#Test methods|extract as discussed by Bläuer]]. A few drops of this extract are placed onto a microscope slide and observed under the polarizing microscope. It is important to observe the crystallization of the salts from the beginning, i.e., from the appearance of the first small crystals until the end of the crystallization process. Only then, the right conclusions can be drawn, because salts that crystallize later may cover up some salts completely, and therefore their crystal shape may become difficult to recognize and to identify. The continuous documentation of the process is therefore an important option. | ||
| Line 25: | Line 27: | ||
== Halite (NaCl) == | == Halite (NaCl) == | ||
Figures 1-3 show halite crystals. Halite and sylvite (KCl) are the | Figures 1-3 show halite crystals. Halite and sylvite (KCl) are the most commonly found isotropic salt crystals, i.e., belonging to the cubic crystal system in building materials. This does not mean that all crystals that appear isotropic on the microscope slide are cubic, like halite and sylvite. On the slide, some salts crystallize at an orientation that make them appear optically isotropic. Therefore caution is vital. In comparison with typical cubic crystal shapes, however, the identification is unambiguous. | ||
<dpl> | <dpl> | ||
| Line 44: | Line 46: | ||
== Calcium chloride == | == Calcium chloride == | ||
Calcium chloride only crystallizes at relatively low relative humidity levels (pure salts at a RH <30, 8% and 20°C). Because the relative humidity on objects and in the laboratory usually lies above this value, | Calcium chloride only crystallizes at relatively low relative humidity levels (pure salts at a RH <30, 8% and 20°C). Because the relative humidity on objects and in the laboratory usually lies above this value, calcium chloride will only rarely crystallize on an object. To have this salt crystallize so as to observe its crystals under the polarizing microscope, the slide with the solution has to be warmed up until they form. However, the crystals dissolve in the ambient air when the temperature cools down and the RH rises. Figures 4- 6 show calcium chloride crystals while warming. | ||
| Line 58: | Line 60: | ||
<gallery caption="Calcium chloride, crystallized from aqueous solution on a microscope slide " widths="200px" heights="150px" perrow="3"> | <gallery caption="Calcium chloride, crystallized from aqueous solution on a microscope slide " widths="200px" heights="150px" perrow="3"> | ||
Image:HJS_Ca%28Cl%292_100903-10-7.jpg| | Image:HJS_Ca%28Cl%292_100903-10-7.jpg|Figure 4: Calcium chloride crystals under polarized light | ||
Image:HJS_Ca%28Cl%292_100903-10-6.jpg| | Image:HJS_Ca%28Cl%292_100903-10-6.jpg|Figure 5: Calcium chloride crystals under polarized light with analyzer | ||
Image:HJS_Ca%28Cl%292_100903-10-1.jpg| | Image:HJS_Ca%28Cl%292_100903-10-1.jpg|Figure 6: Calcium chloride crystals under polarized light with analyzer and red I | ||
</gallery> | </gallery> | ||
| Line 197: | Line 199: | ||
<gallery caption=" Sodium acetate crystallized from aqueous solution" widths="200px" heights="150px" perrow="3"> | <gallery caption=" Sodium acetate crystallized from aqueous solution" widths="200px" heights="150px" perrow="3"> | ||
Image: Natriumacetat_2010.JPG| Figure 35: Sodium acetate crystals under polarized light | Image: Natriumacetat_2010.JPG| Figure 35: Sodium acetate crystals under polarized light | ||
Image: Natriumacetat_2010 (1).JPG Figure 36: Sodium acetate crystals under polarized light, detail Figure 35 | Image: Natriumacetat_2010 (1).JPG| Figure 36: Sodium acetate crystals under polarized light, detail Figure 35 | ||
Image: Natriumacetat_2010 (2).JPG| Figure 37: Sodium acetate crystals under polarized light with analyzer and red I, detail Figure 35 | Image: Natriumacetat_2010 (2).JPG| Figure 37: Sodium acetate crystals under polarized light with analyzer and red I, detail Figure 35 | ||
</gallery> | </gallery> | ||
| Line 206: | Line 208: | ||
[[Category:Light Microscopy]] [[Category:Schwarz,Hans-Jürgen]] [[Category:R-MSteiger]] [[Category:R-CBlaeuer]] [[Category: | [[Category:Light Microscopy]] [[Category:Schwarz,Hans-Jürgen]] [[Category:R-MSteiger]] [[Category:R-CBlaeuer]] [[Category:editing]] | ||
Latest revision as of 12:30, 25 June 2016
Author: Hans-Jürgen Schwarz
English Translation by Sandra Leithäuser
back to Polarized light microscopy or Analysis of Salts
Abstract[edit]
This section describes the identification of salts using polarized light microscopy.
Method[edit]
The examination of salts can be carried out using several different methods, such as microscopy, spot test analysis, X-ray diffraction. It is important to determine both the anions and cations in the salt(s) and, if possible, the specific phases of the actual salts. In general, laboratories carrying out routine chemical analysis do not usually determine the presence of carbonate ions, and therefore this salt is not identified, although they are often present and can be responsible for the observed efflorescence and eventual deterioration.
Two different approaches to microscopic analysis of salts are presented here:
The first approach considers mainly the analysis of the salts, that is, the salt crystals actually taken from the object.
The second approach analyses the recrystallized salts obtained from the aqueous extraction of either the salts themselves or of a sample of the salt-contaminated material. Both possibilities lead to salt crystallizations that vary, depending on the different kind of salt, as described below. See also Micro-chemical testing.
Determination of individual salts in aqueous extract[edit]
Before the actual determination of salts, the sample to be examined (pure salt or material/salt mixture) is mixed with a few drops of distilled water, producing an aqueous extract as discussed by Bläuer. A few drops of this extract are placed onto a microscope slide and observed under the polarizing microscope. It is important to observe the crystallization of the salts from the beginning, i.e., from the appearance of the first small crystals until the end of the crystallization process. Only then, the right conclusions can be drawn, because salts that crystallize later may cover up some salts completely, and therefore their crystal shape may become difficult to recognize and to identify. The continuous documentation of the process is therefore an important option.
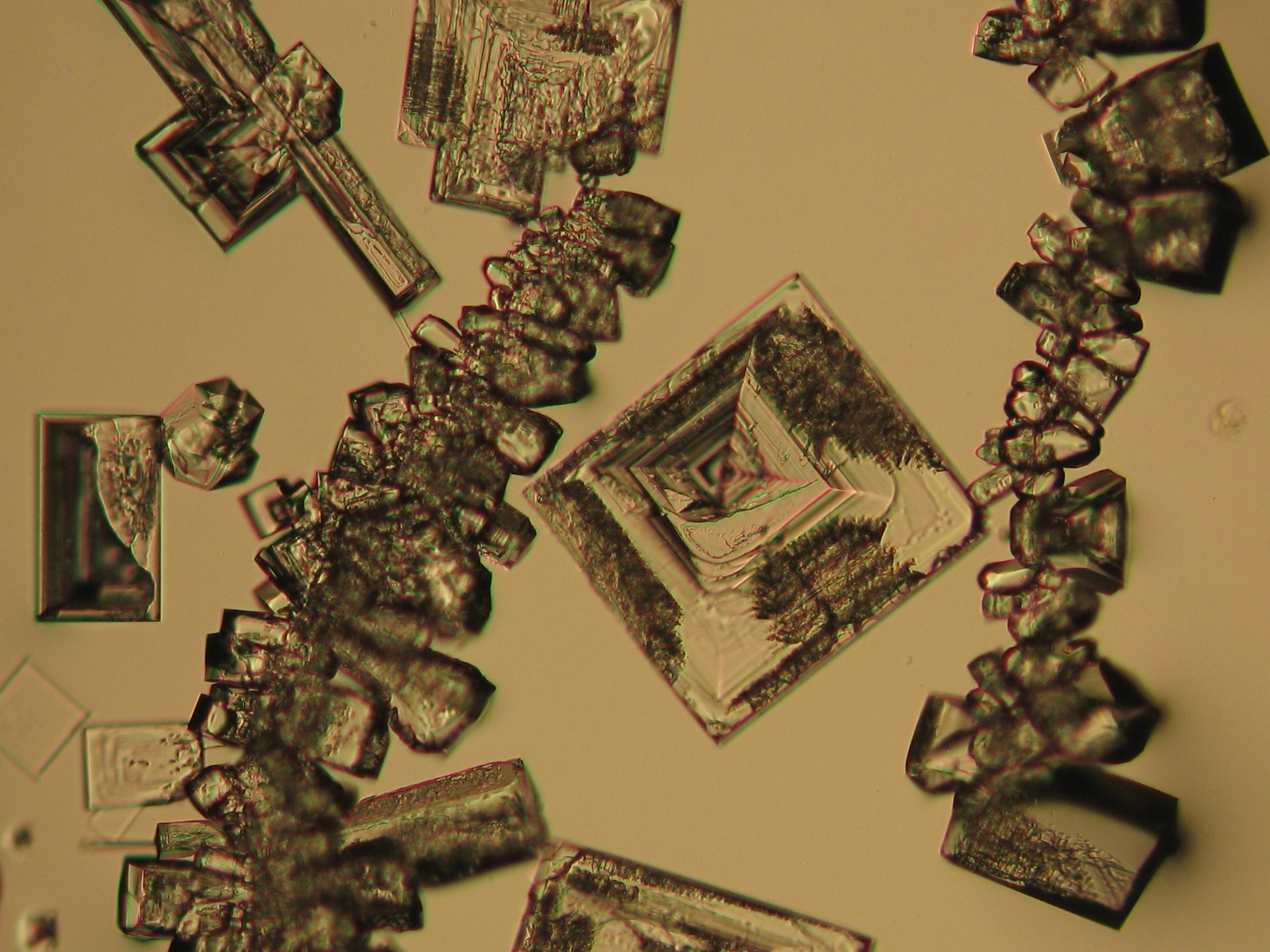
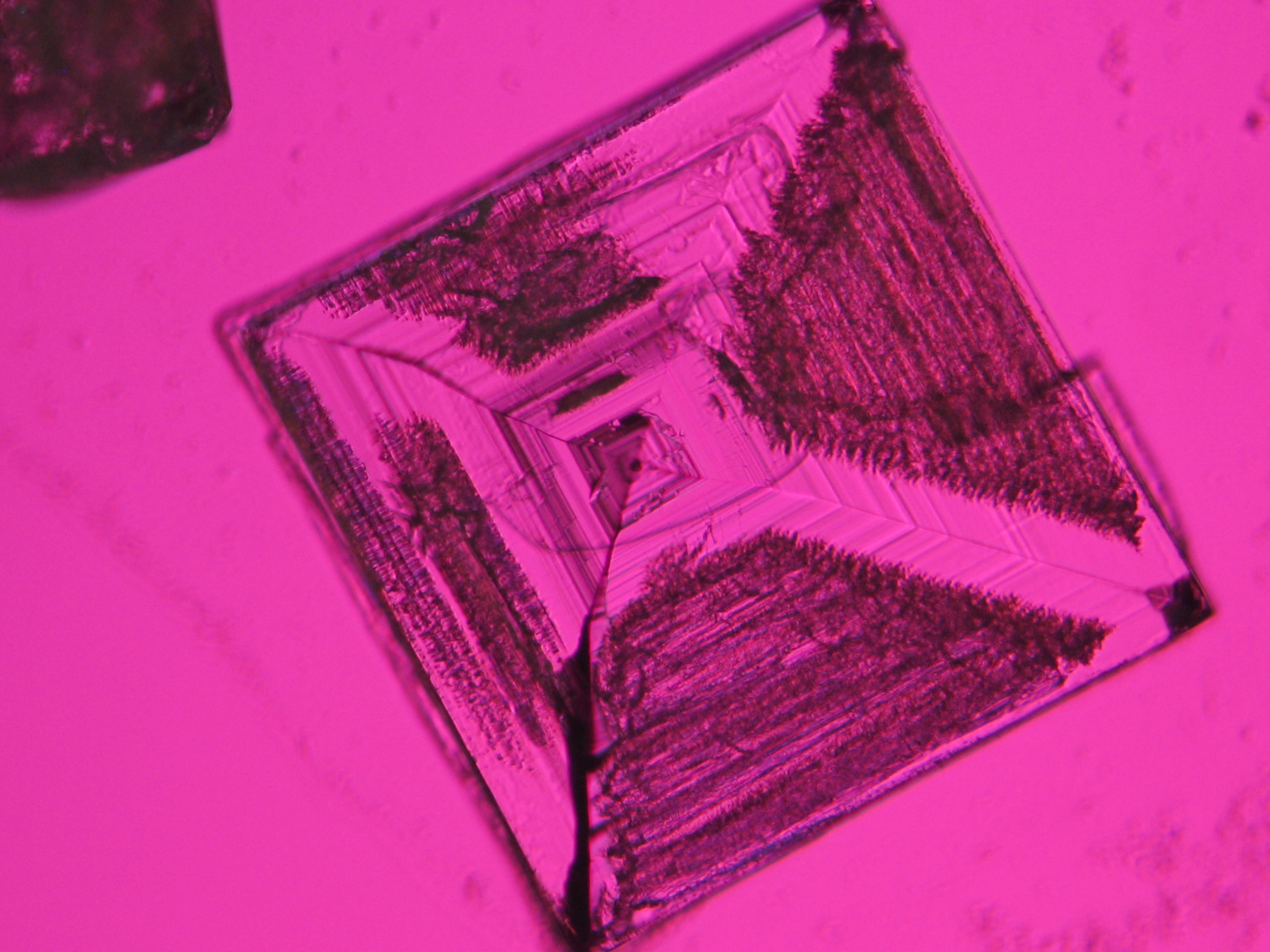
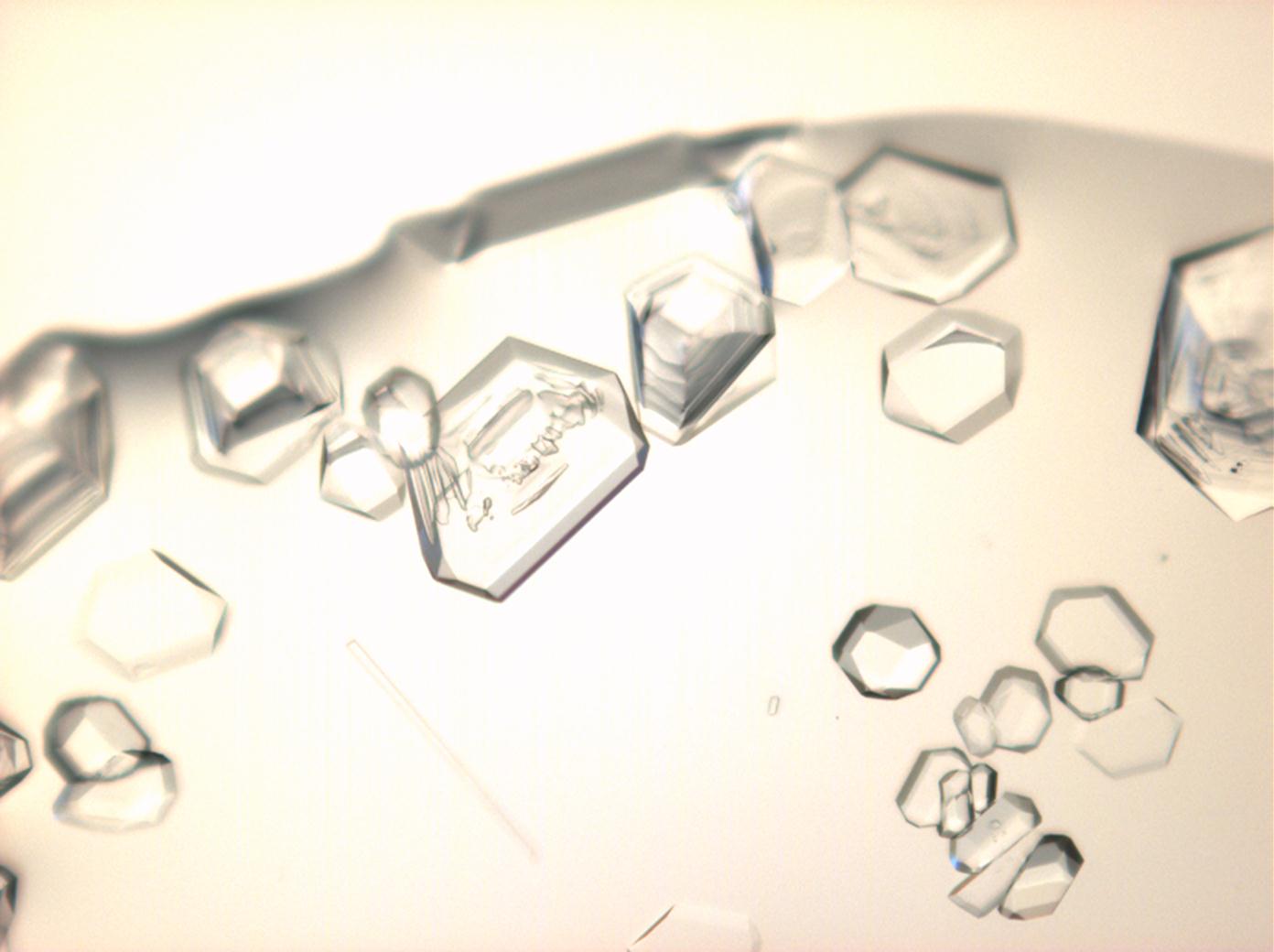
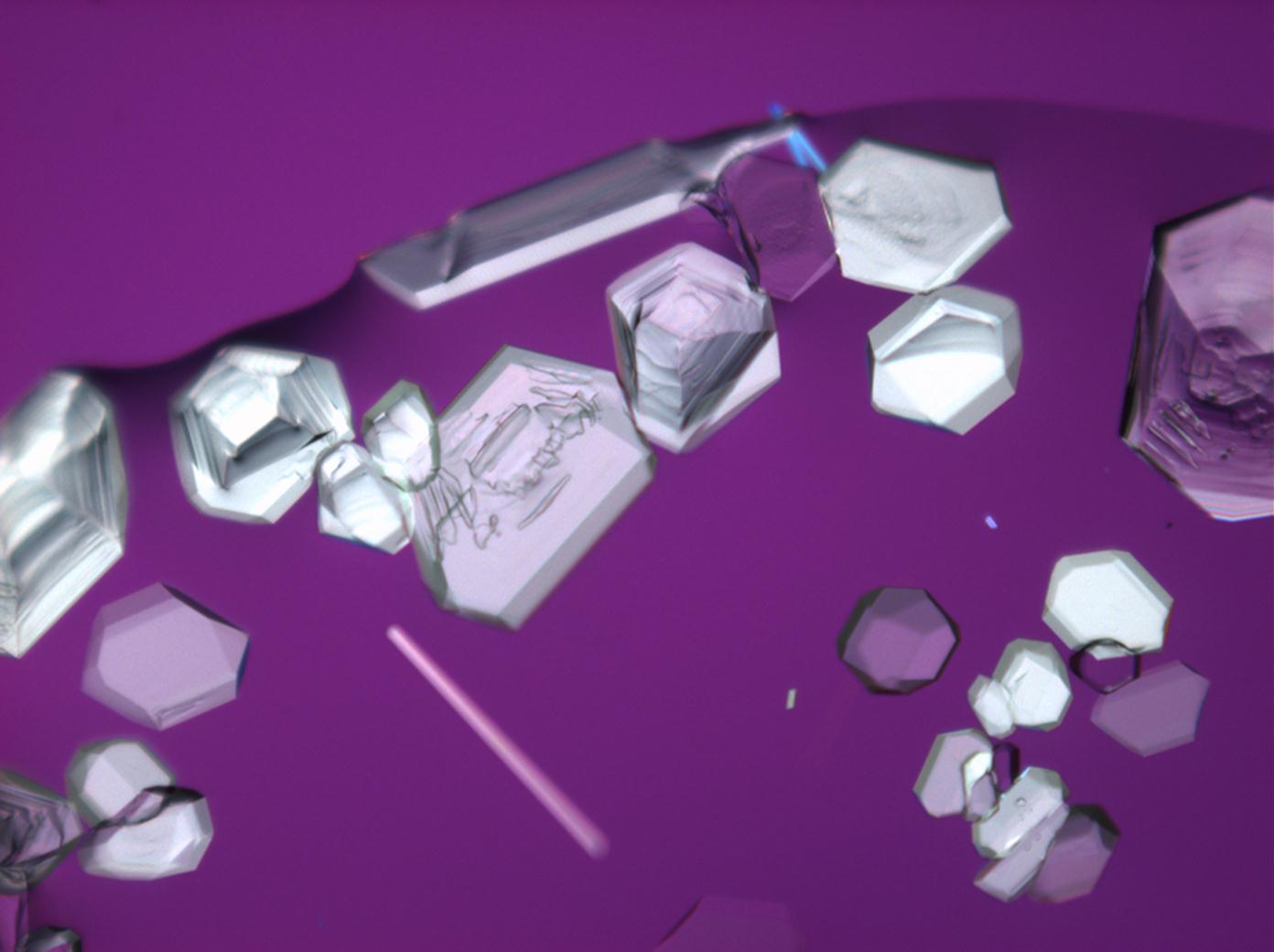
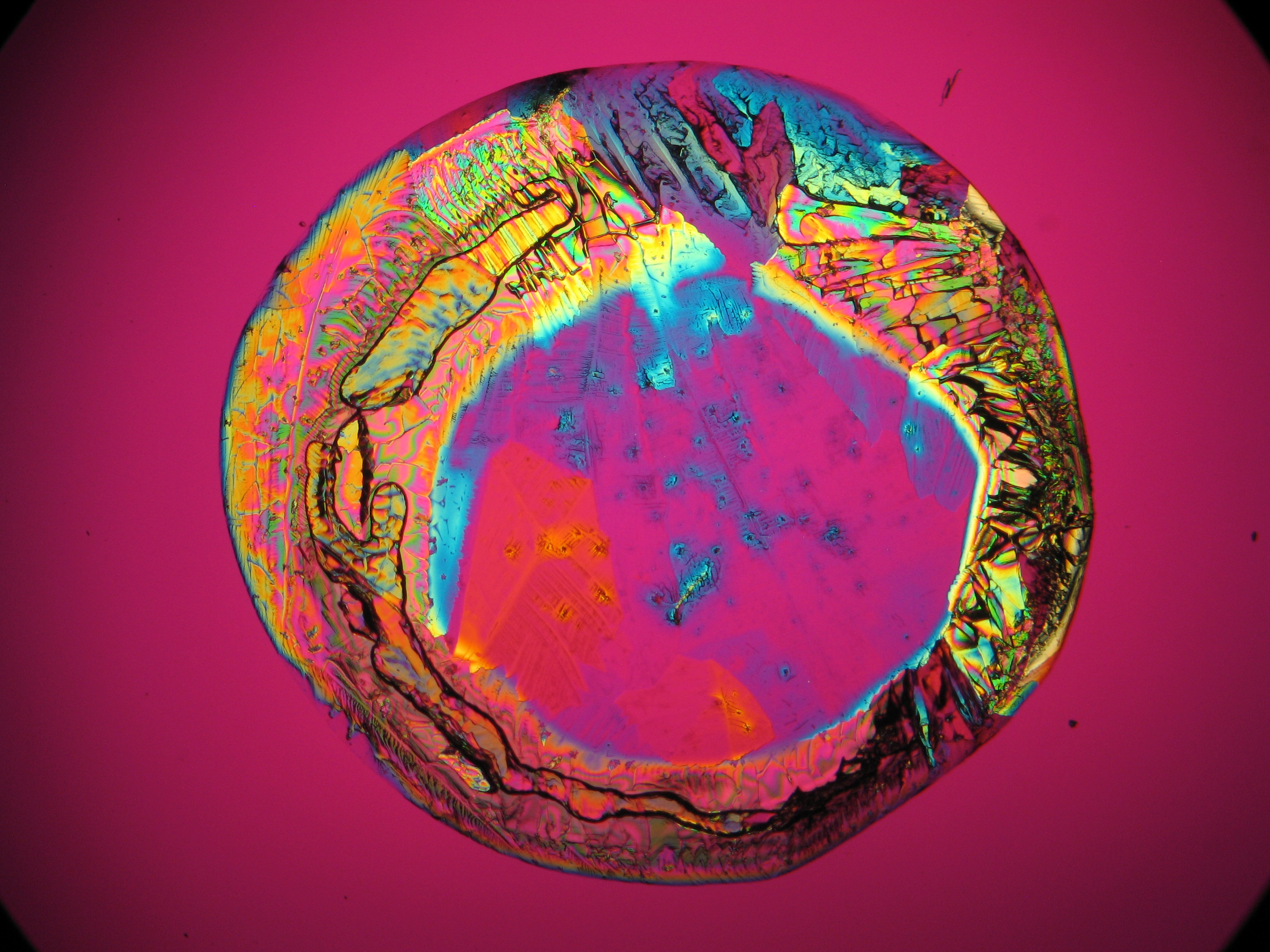
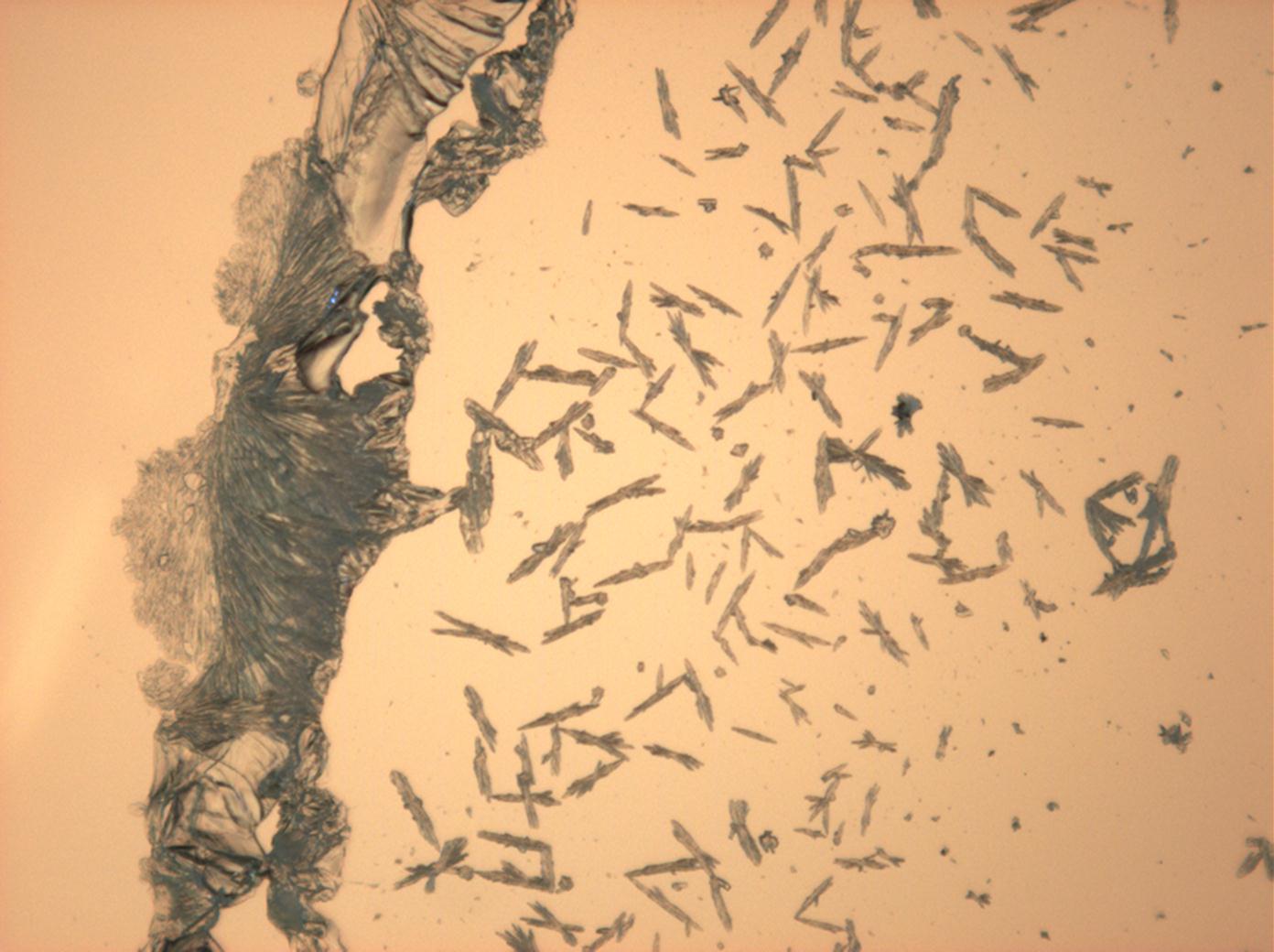
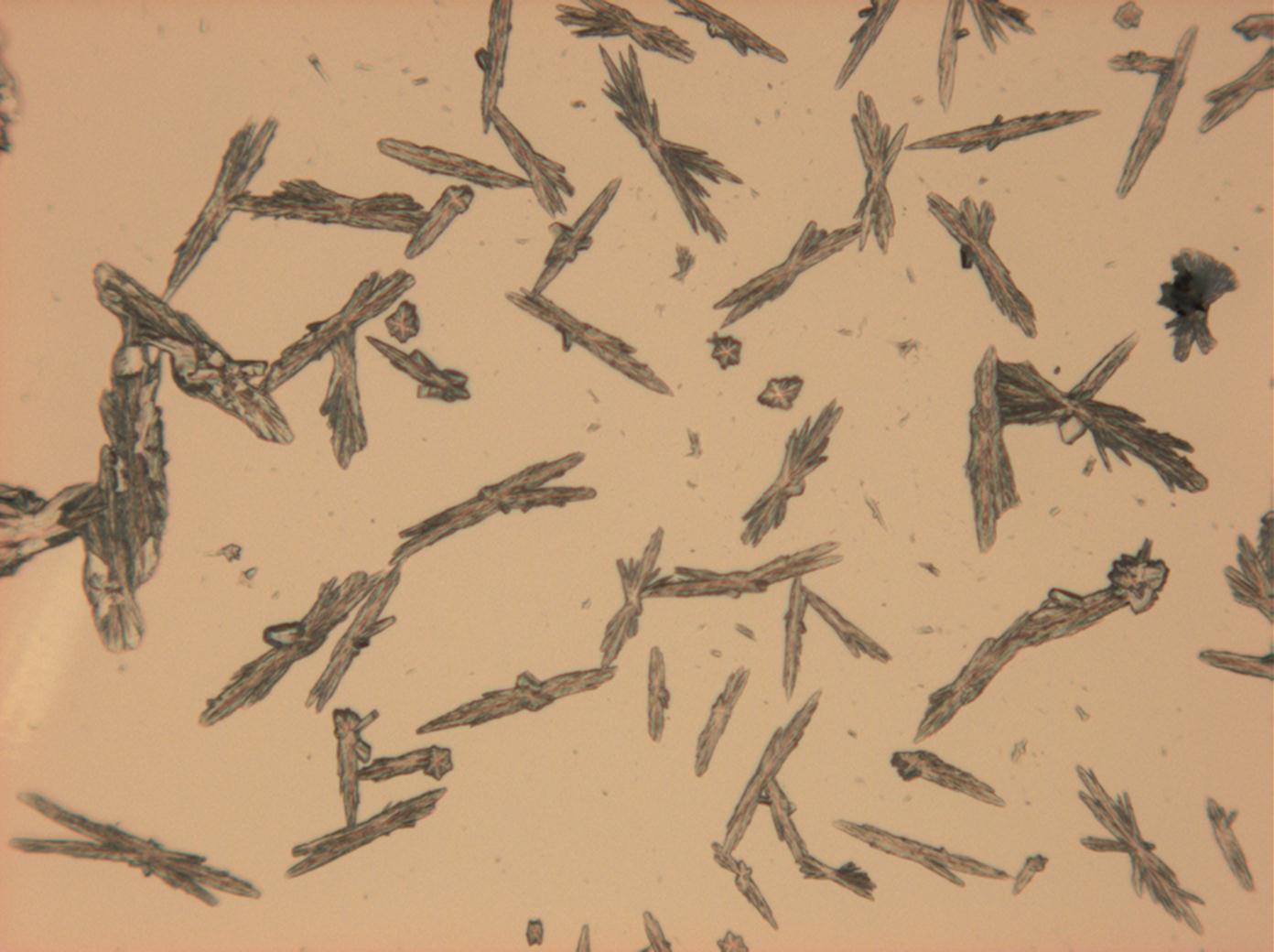
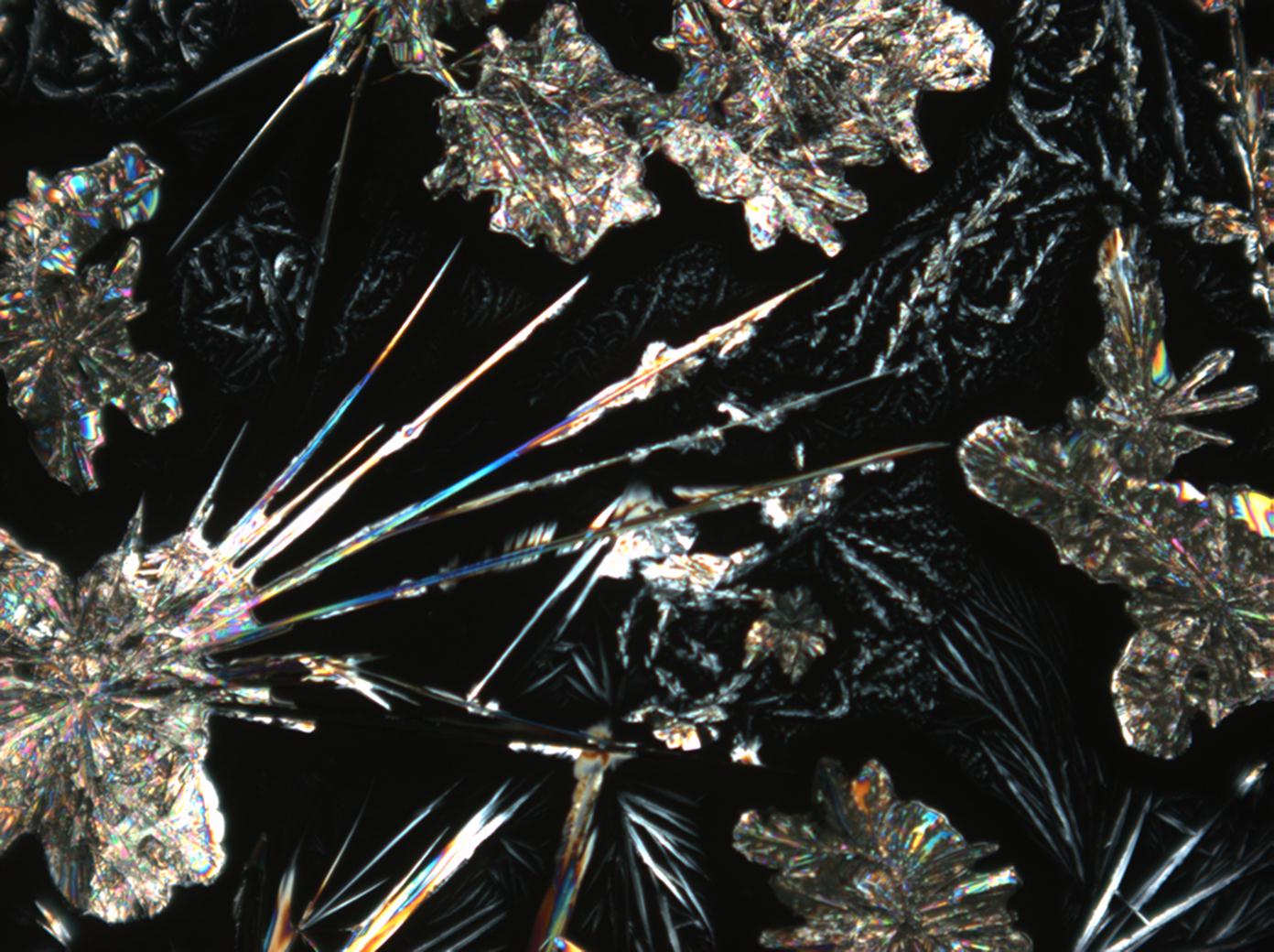
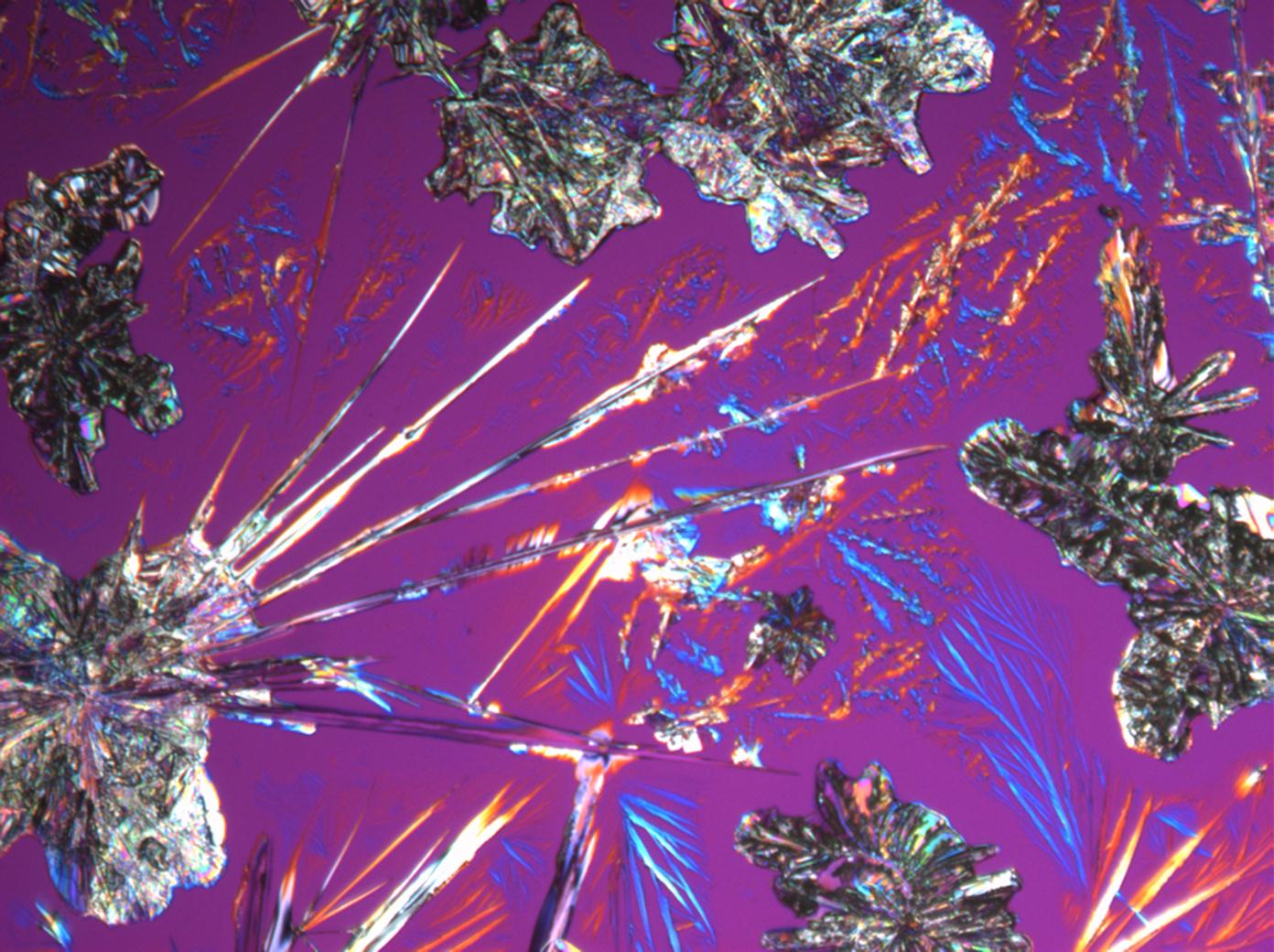
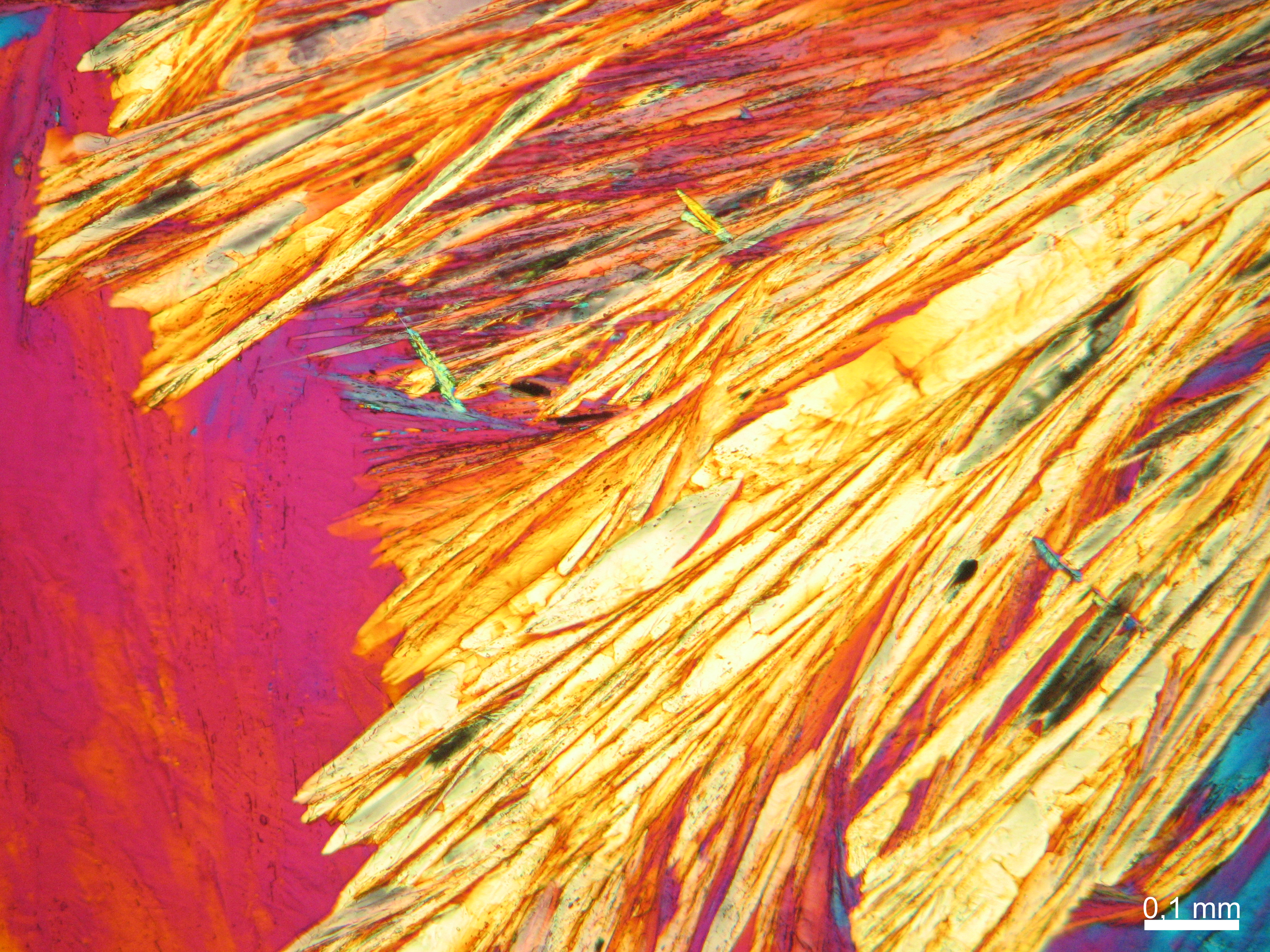
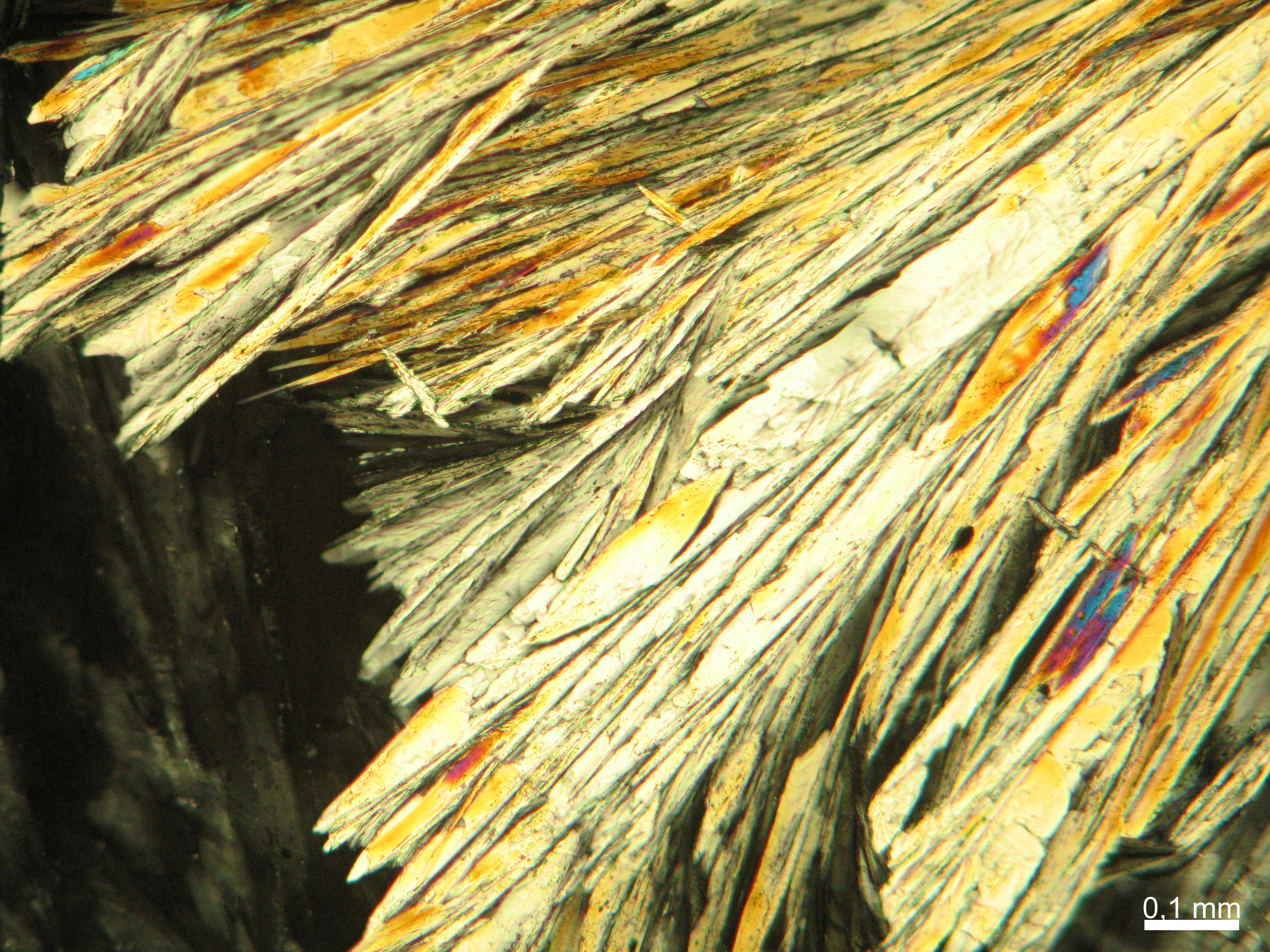
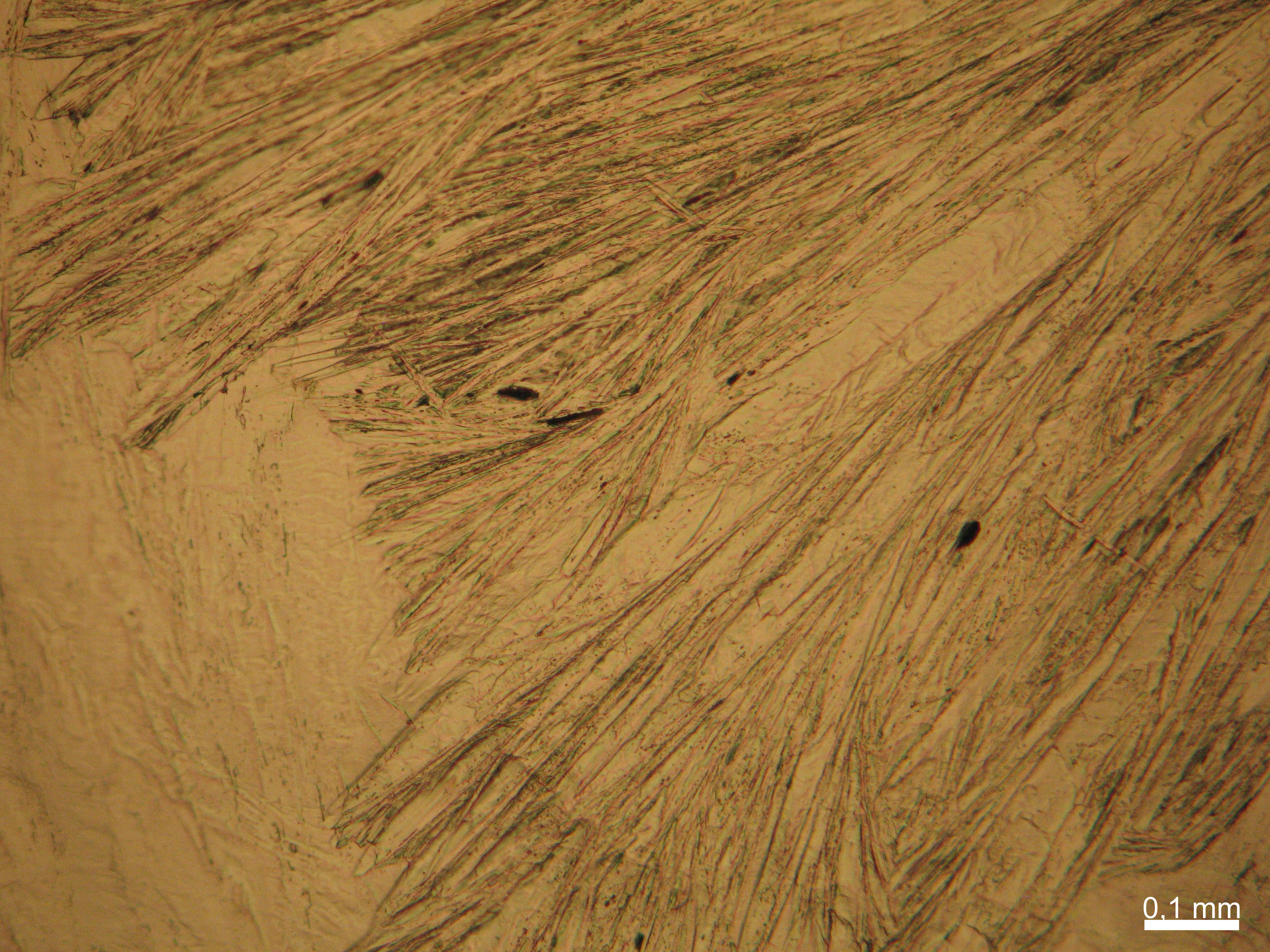
Halite (NaCl)[edit]
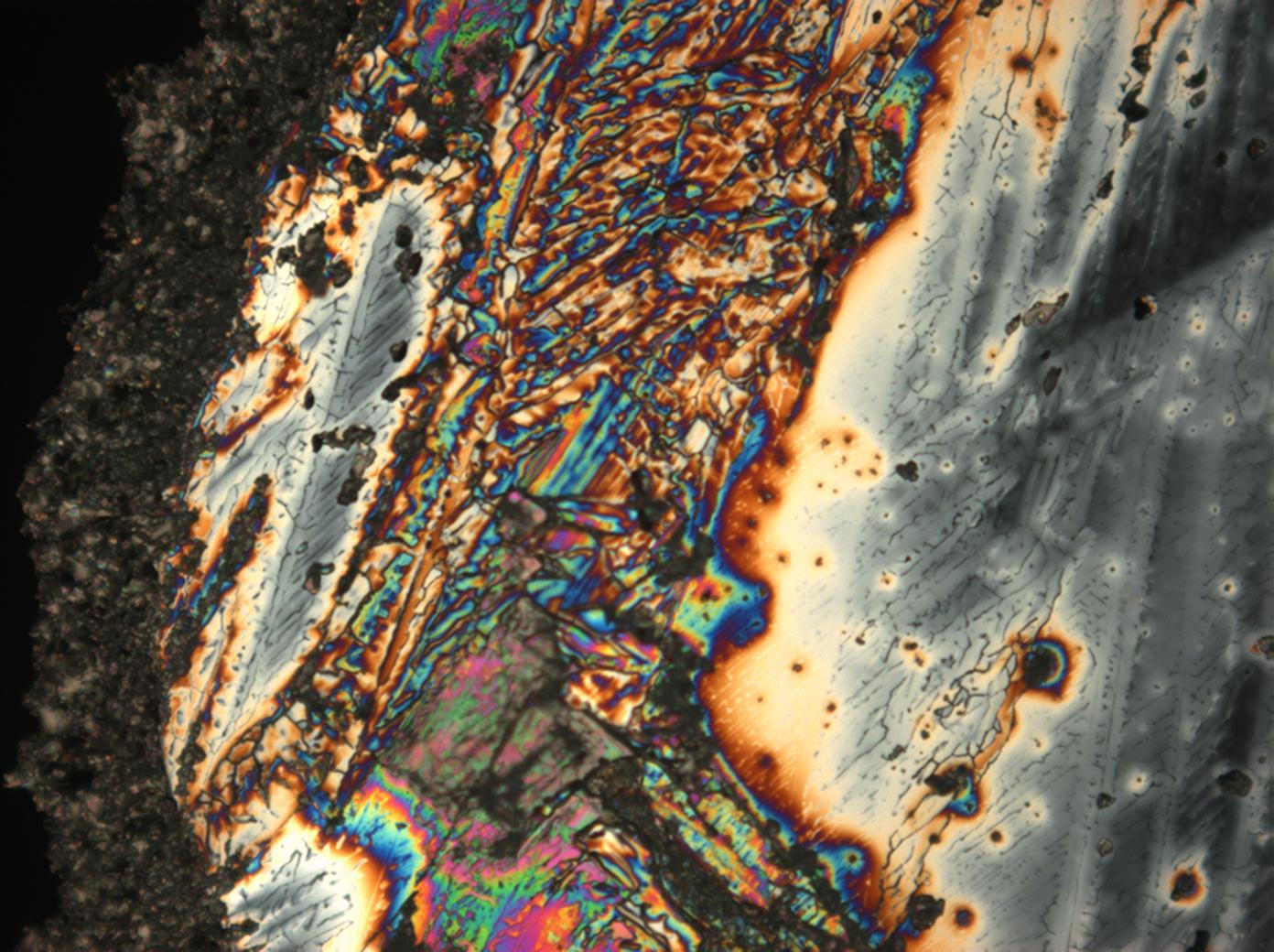
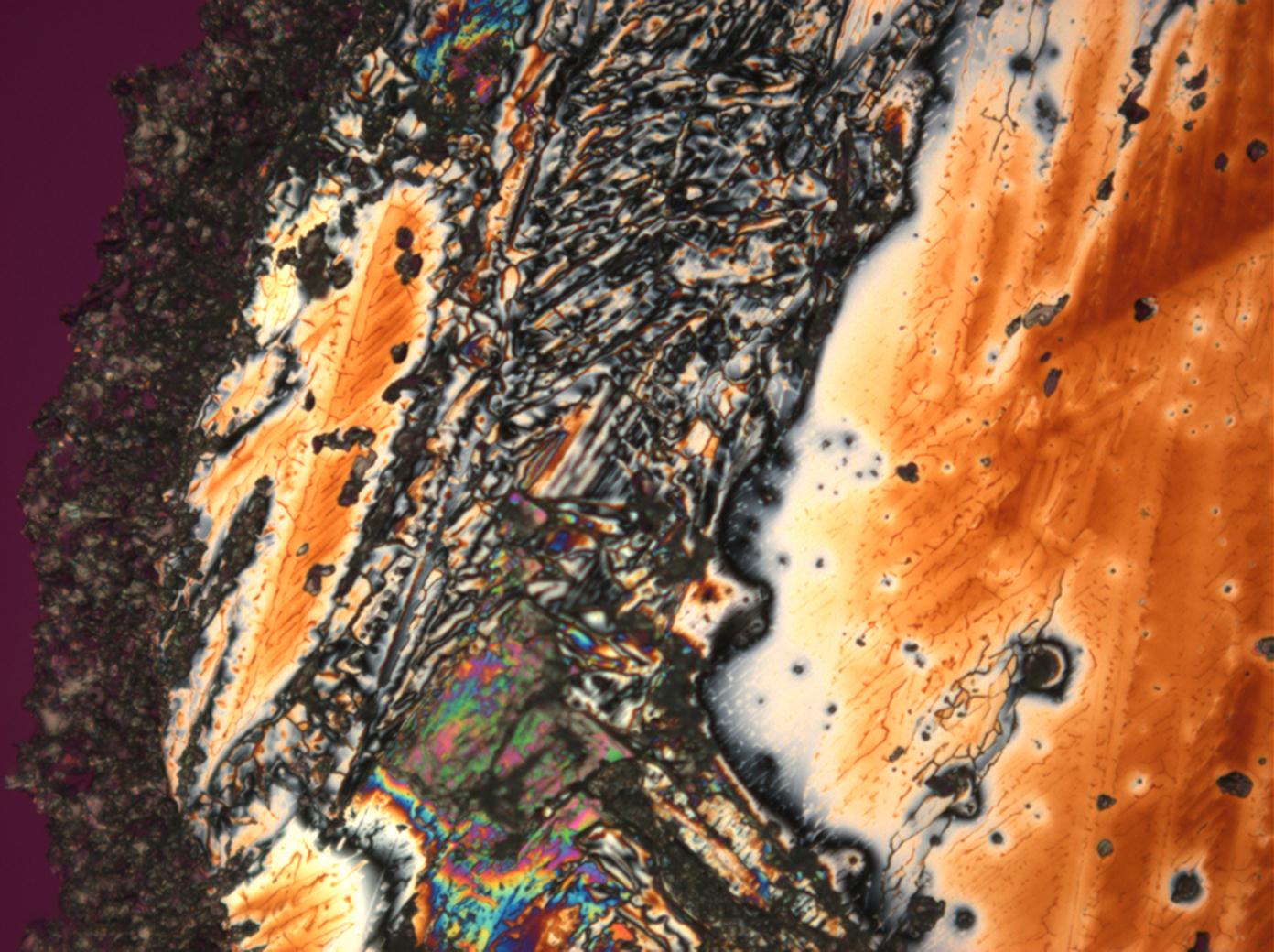
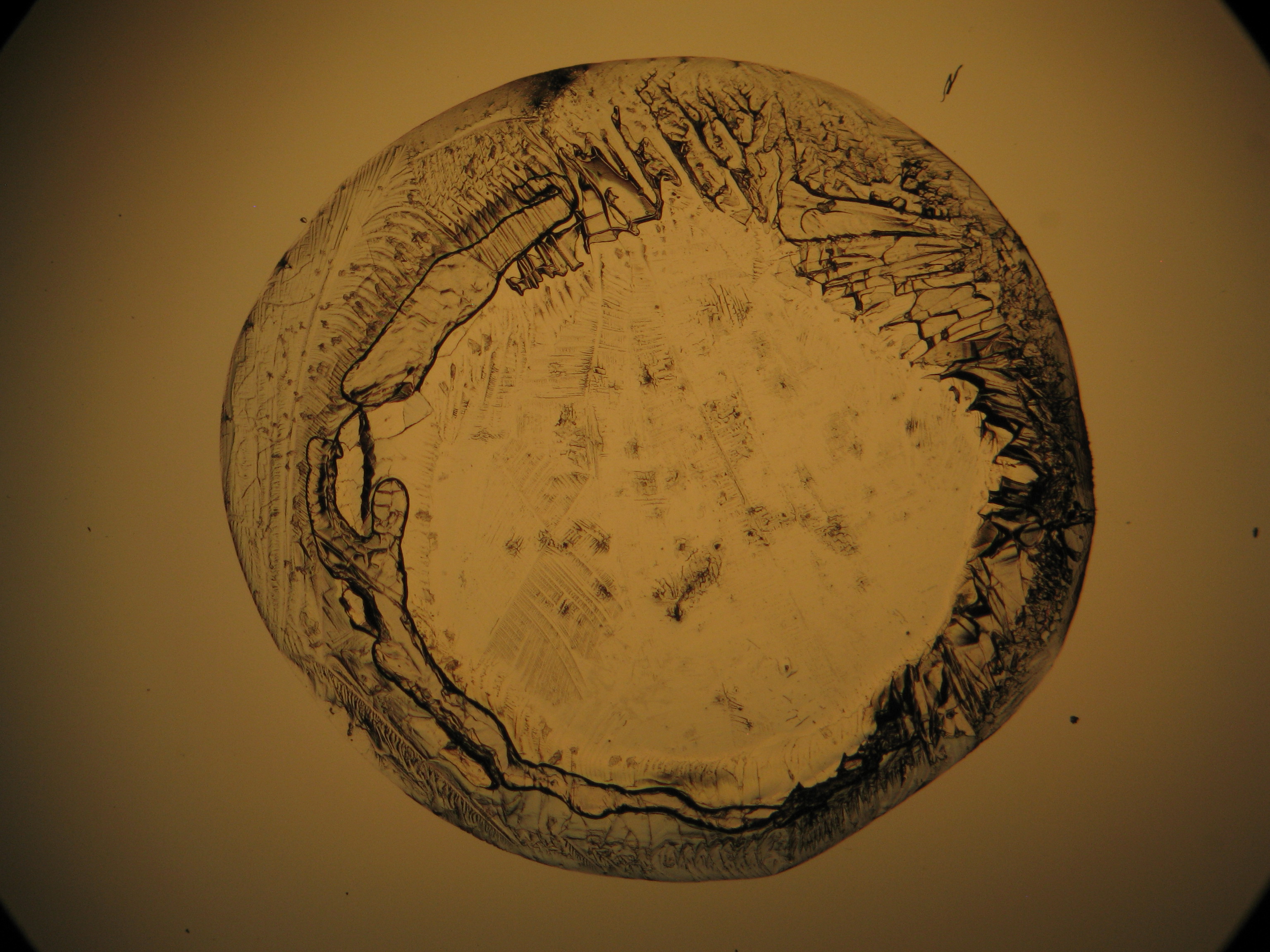
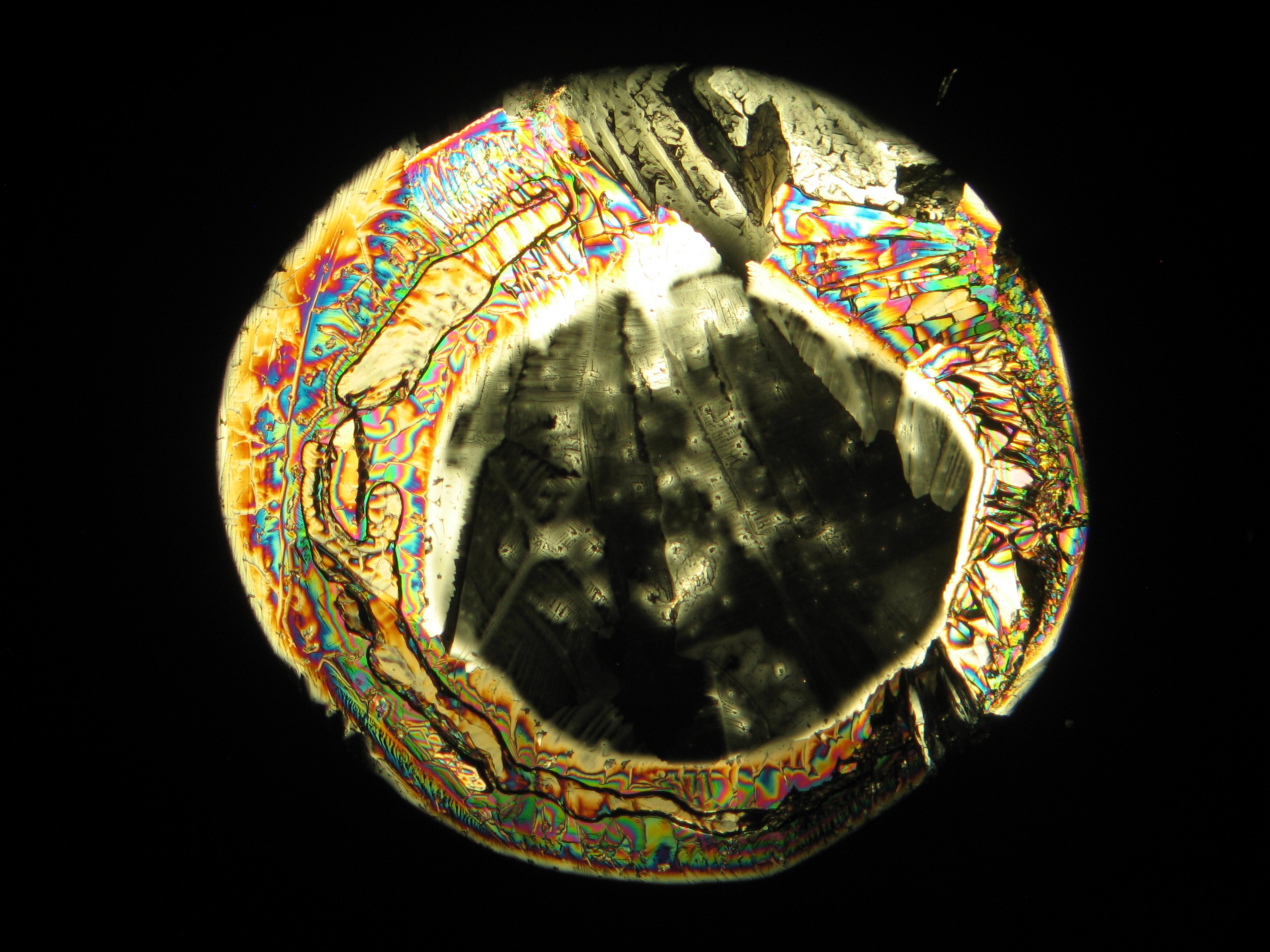
Figures 1-3 show halite crystals. Halite and sylvite (KCl) are the most commonly found isotropic salt crystals, i.e., belonging to the cubic crystal system in building materials. This does not mean that all crystals that appear isotropic on the microscope slide are cubic, like halite and sylvite. On the slide, some salts crystallize at an orientation that make them appear optically isotropic. Therefore caution is vital. In comparison with typical cubic crystal shapes, however, the identification is unambiguous.
| Salt | chemical formula | Birefringence | Refractive indices | Crystal system | Optical orientation |
|---|---|---|---|---|---|
| Halite | NaCl | nD=1.5443 | cubic | isotropic |
- Halite, crystallized from aqueous solution on a microscope slide
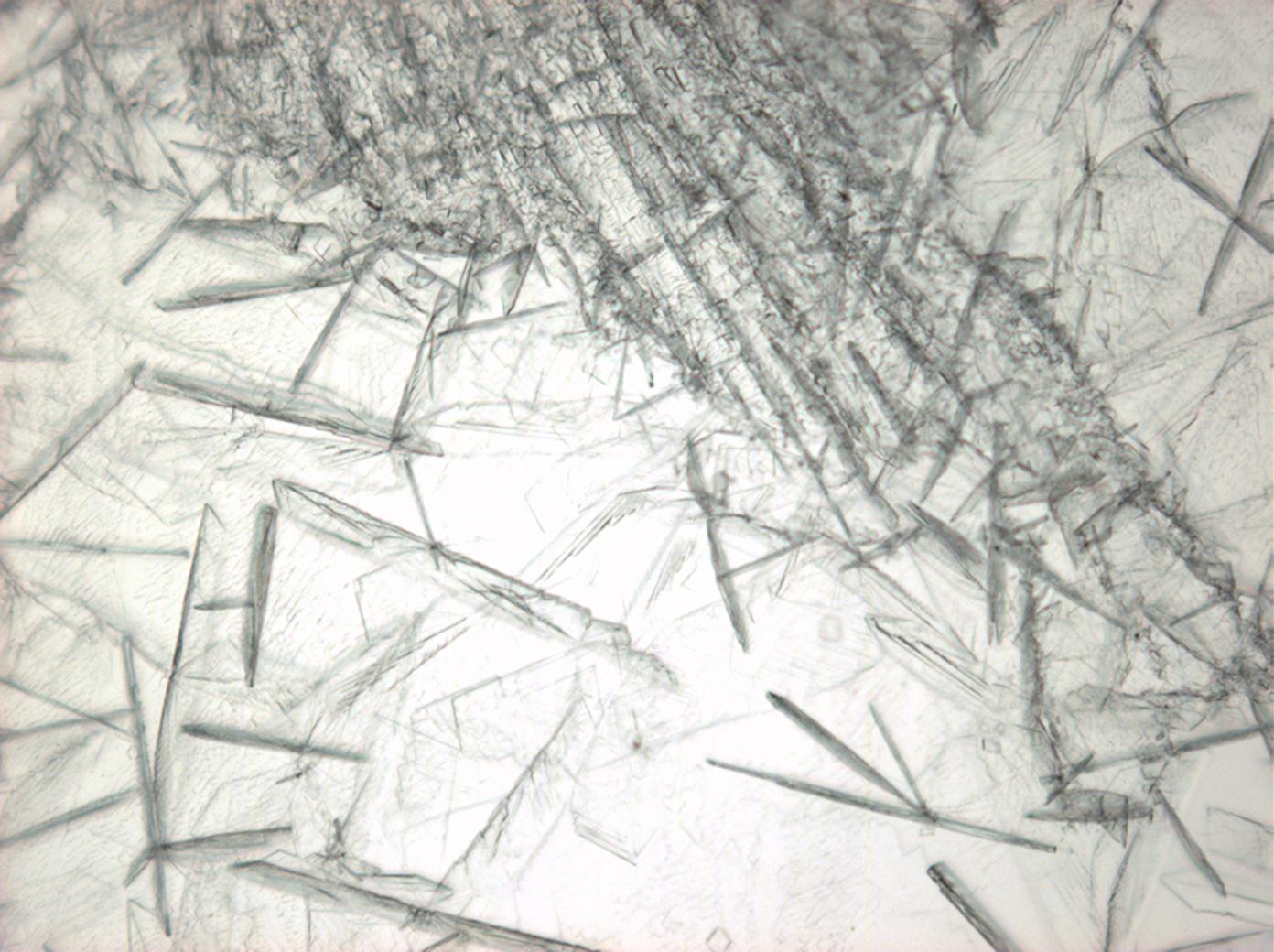
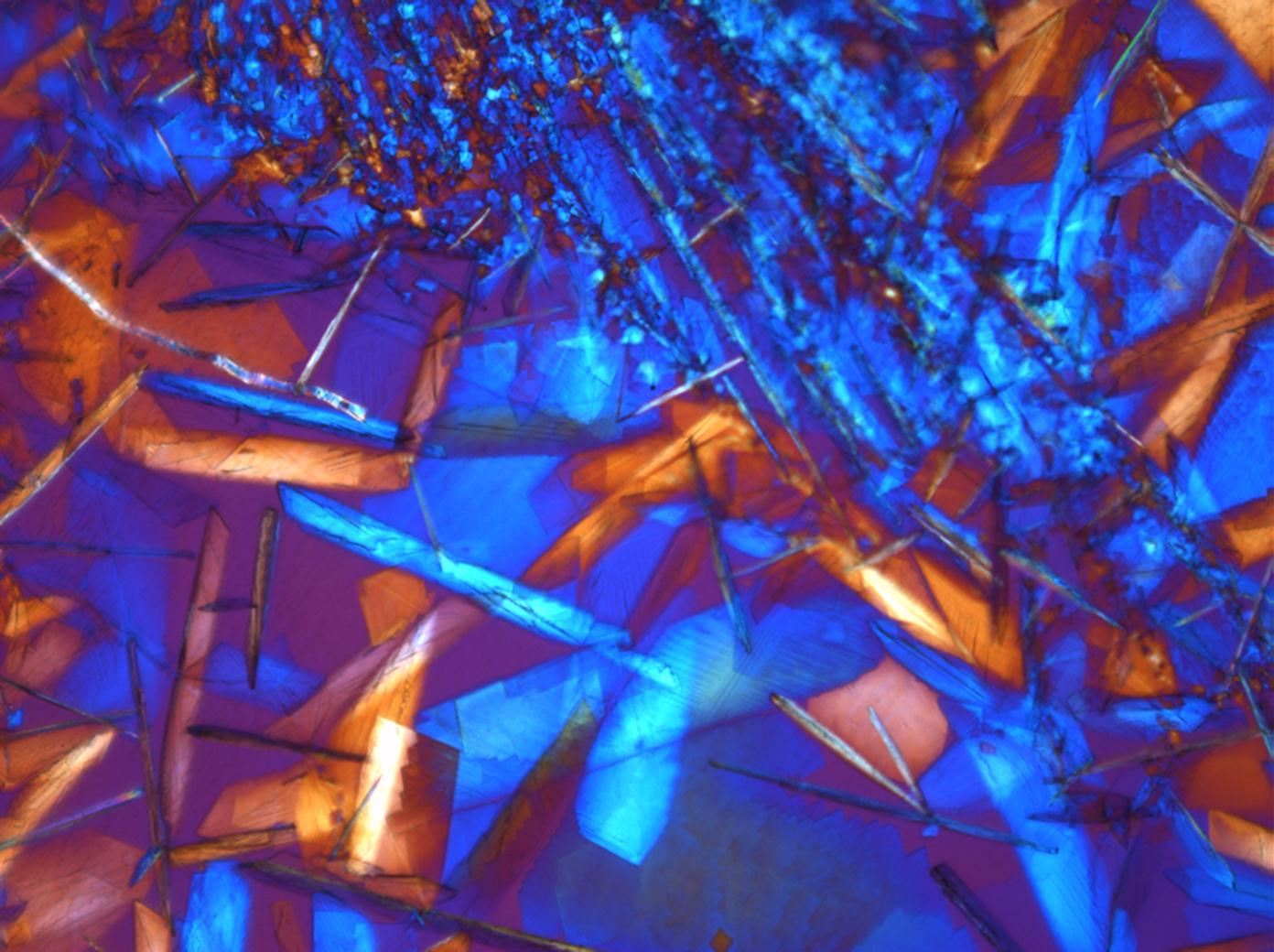
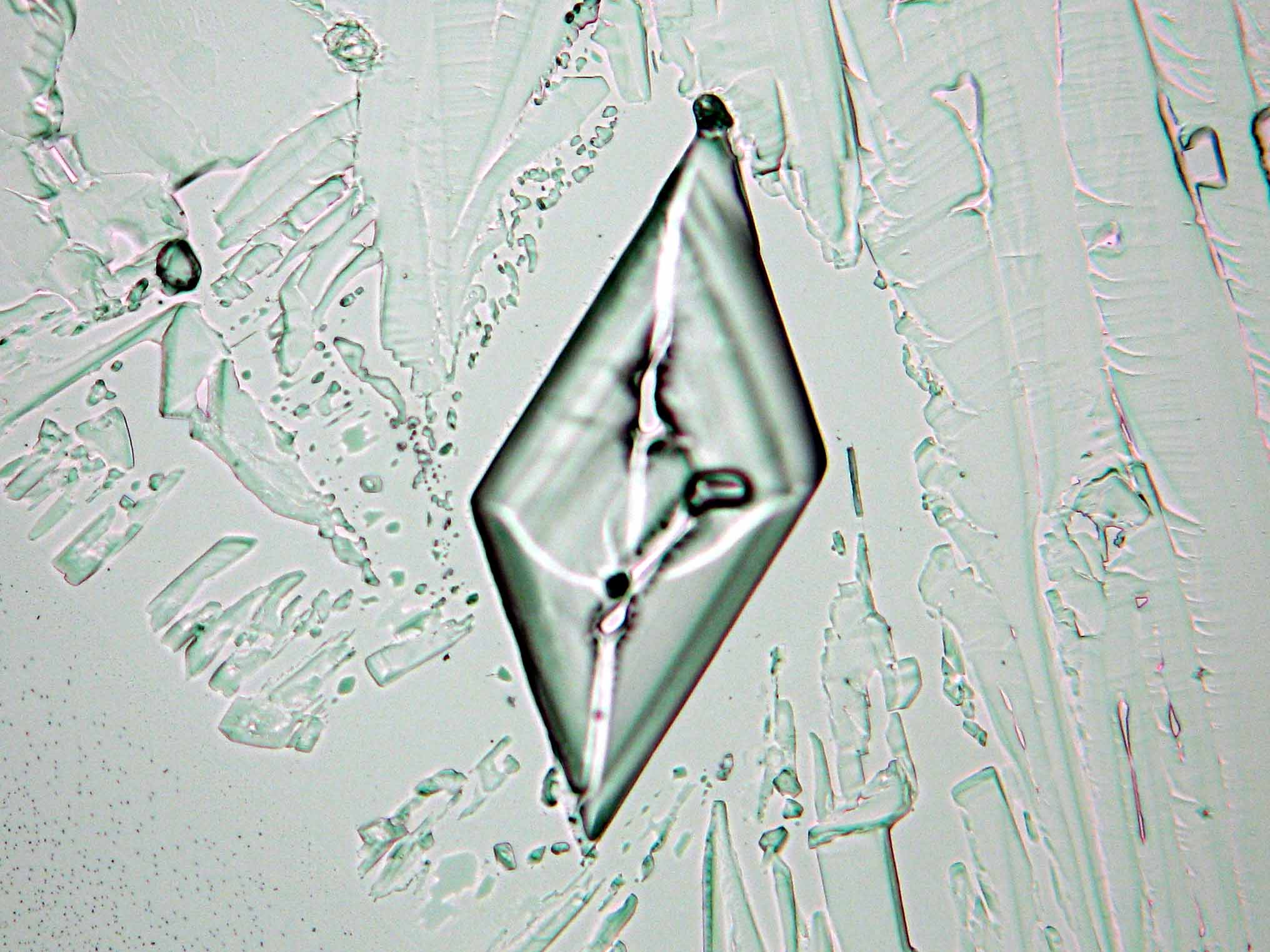
Calcium chloride[edit]
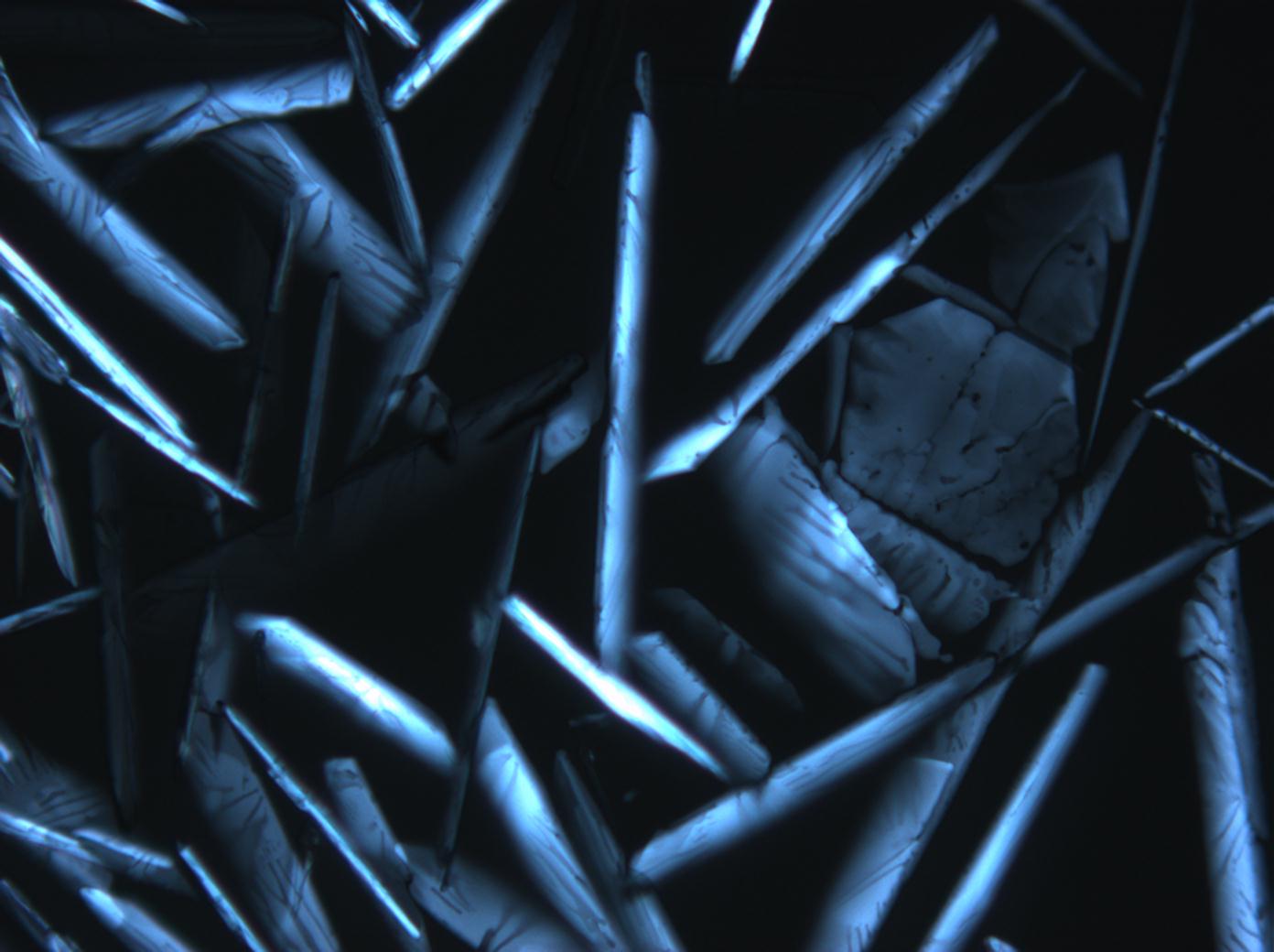
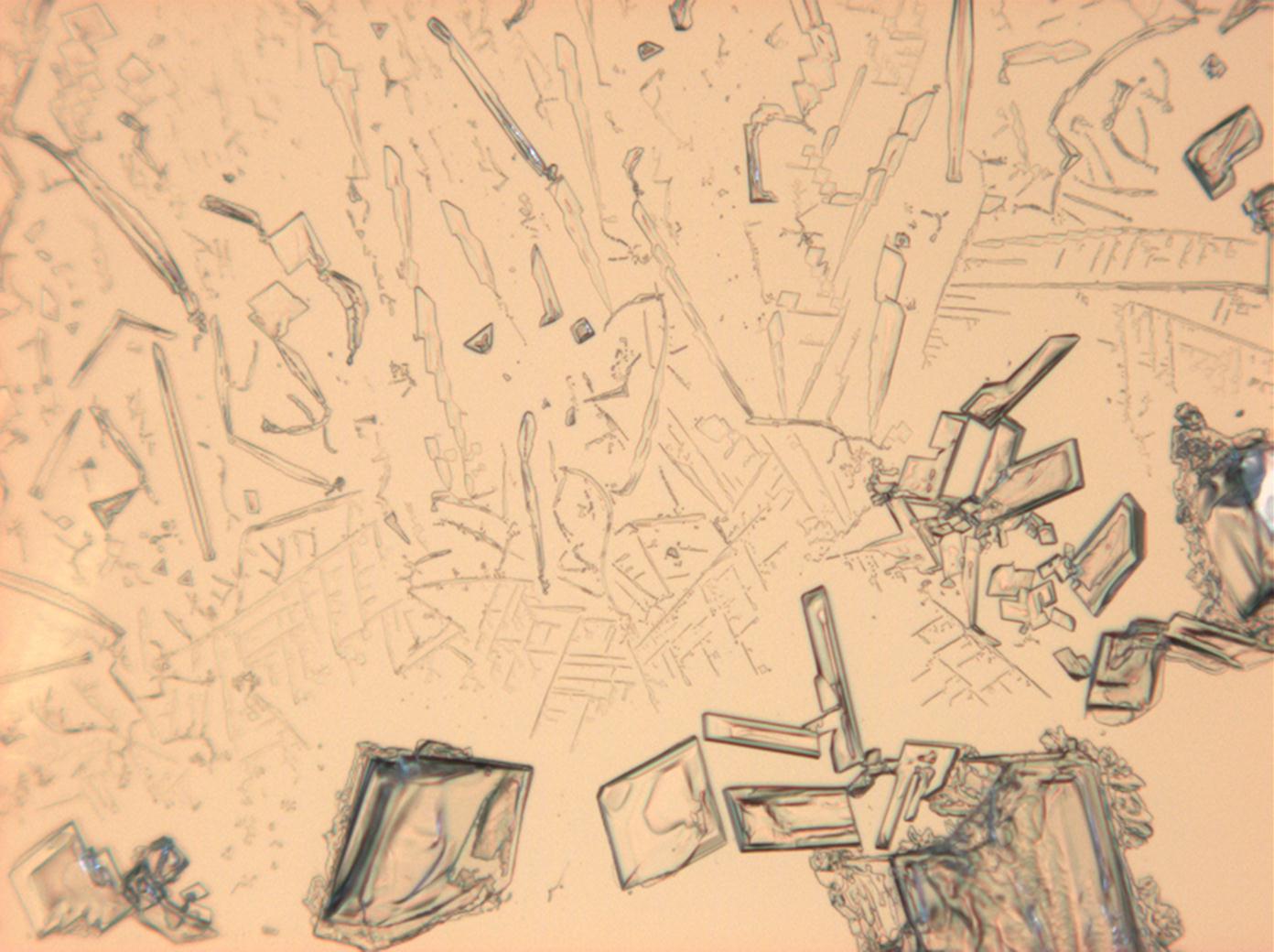
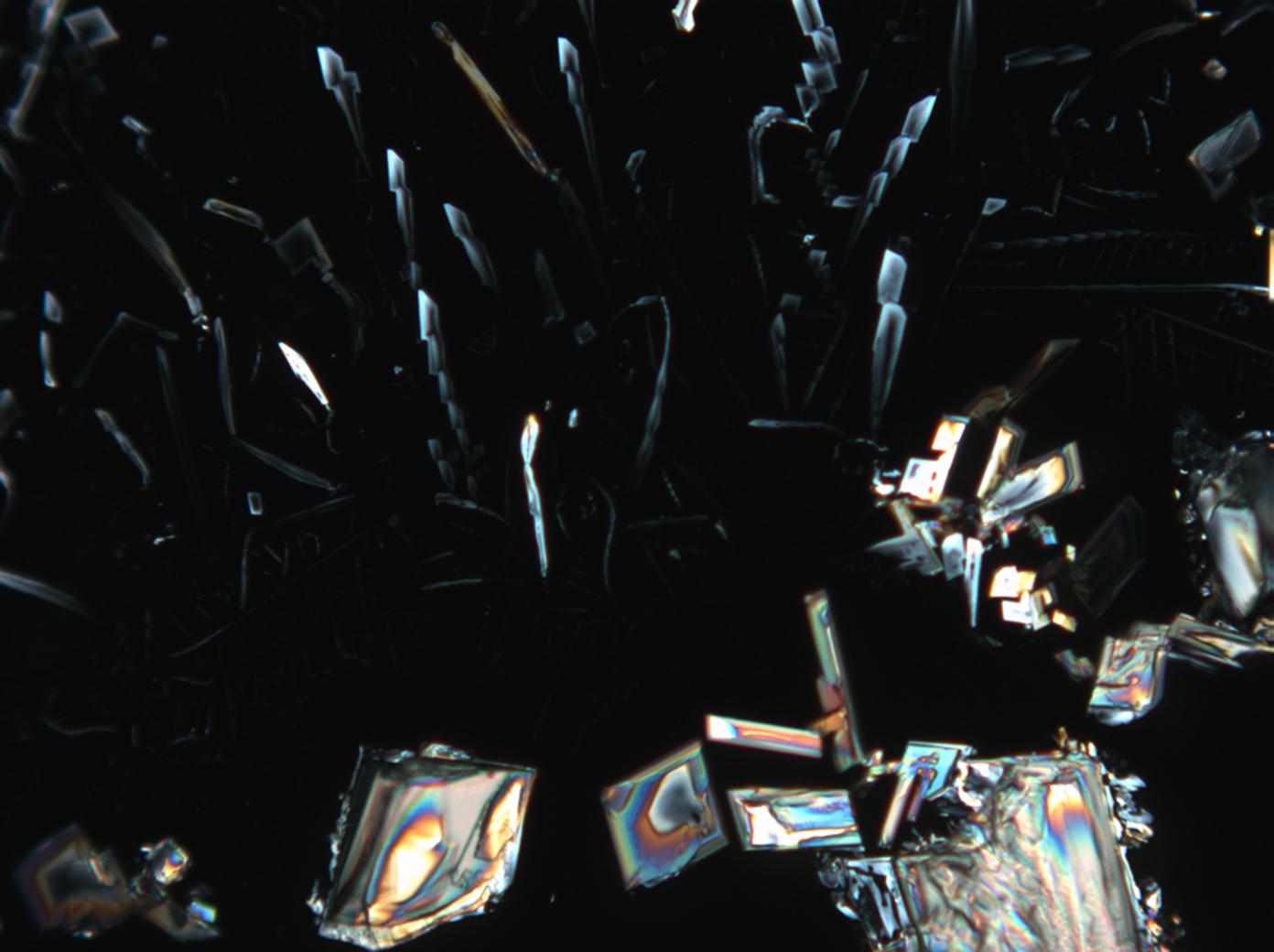
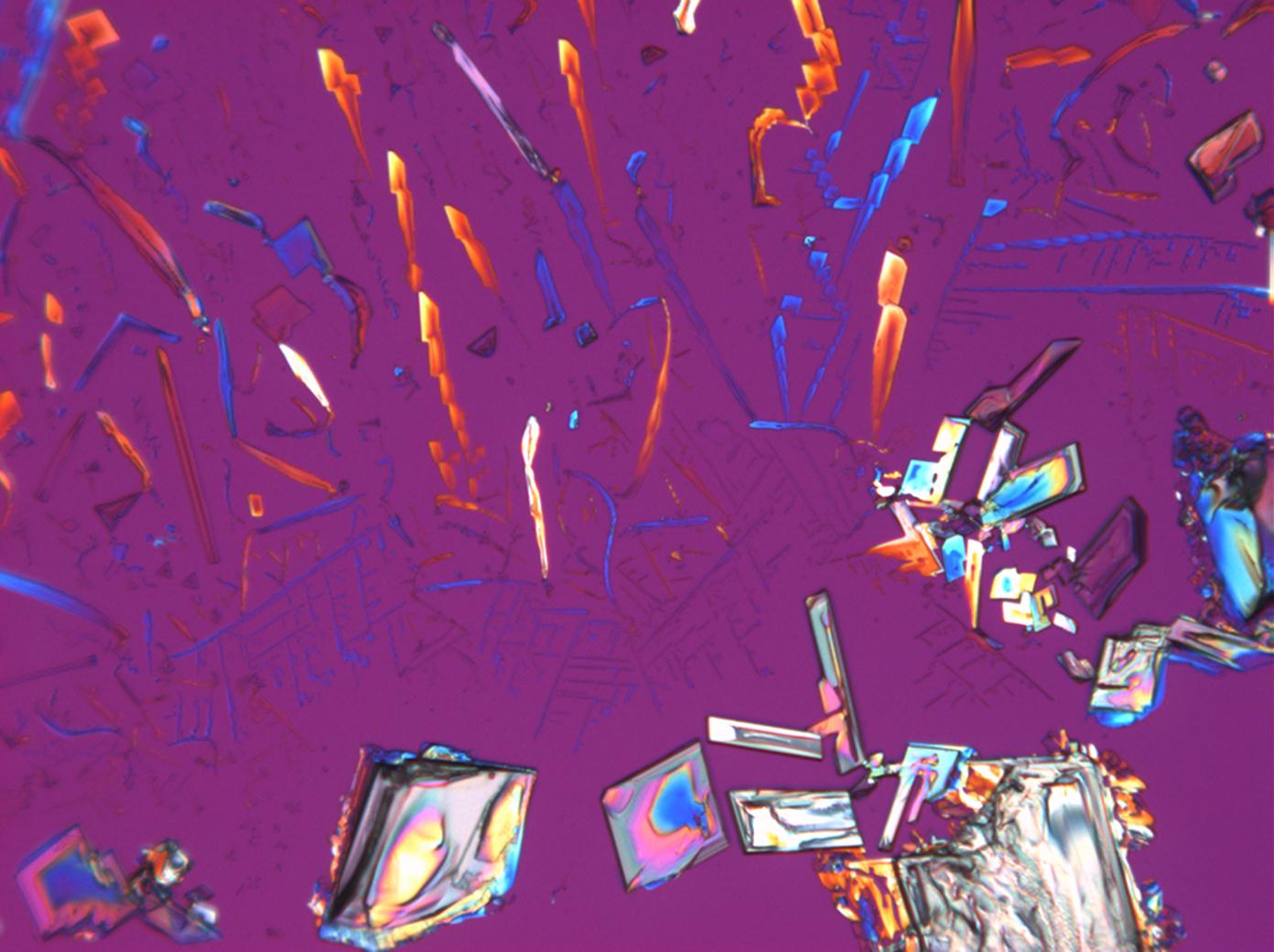
Calcium chloride only crystallizes at relatively low relative humidity levels (pure salts at a RH <30, 8% and 20°C). Because the relative humidity on objects and in the laboratory usually lies above this value, calcium chloride will only rarely crystallize on an object. To have this salt crystallize so as to observe its crystals under the polarizing microscope, the slide with the solution has to be warmed up until they form. However, the crystals dissolve in the ambient air when the temperature cools down and the RH rises. Figures 4- 6 show calcium chloride crystals while warming.
| Salt | chemical formula | Birefringence | Refractive indices | Crystal system | Optical orientation |
|---|---|---|---|---|---|
| Antarcticite | CaCl2•6H2O | Δ= 0.024 | no =1.417-1.494 ne = 1.393-1.550 |
trigonal | negative |
- Calcium chloride, crystallized from aqueous solution on a microscope slide
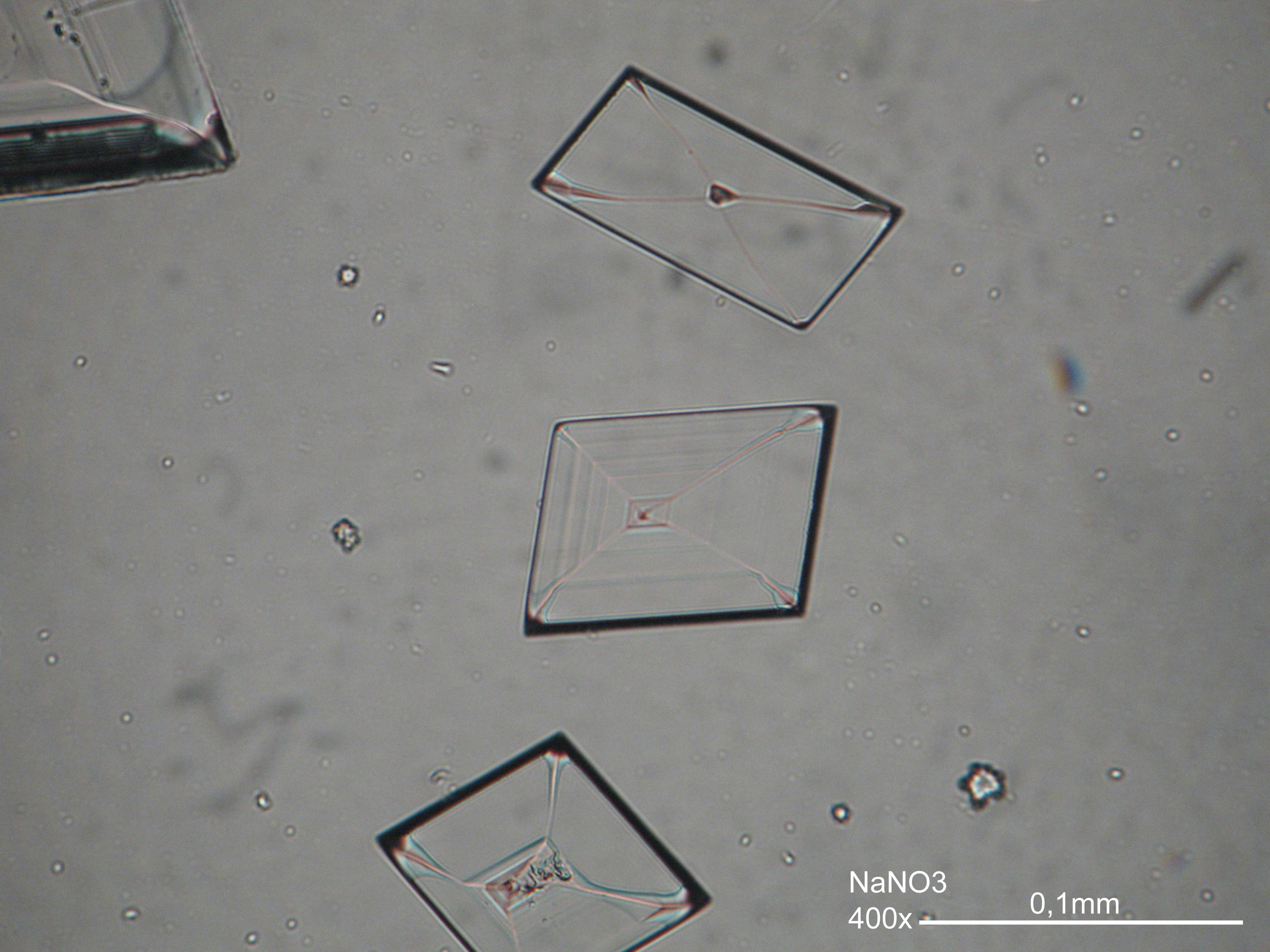
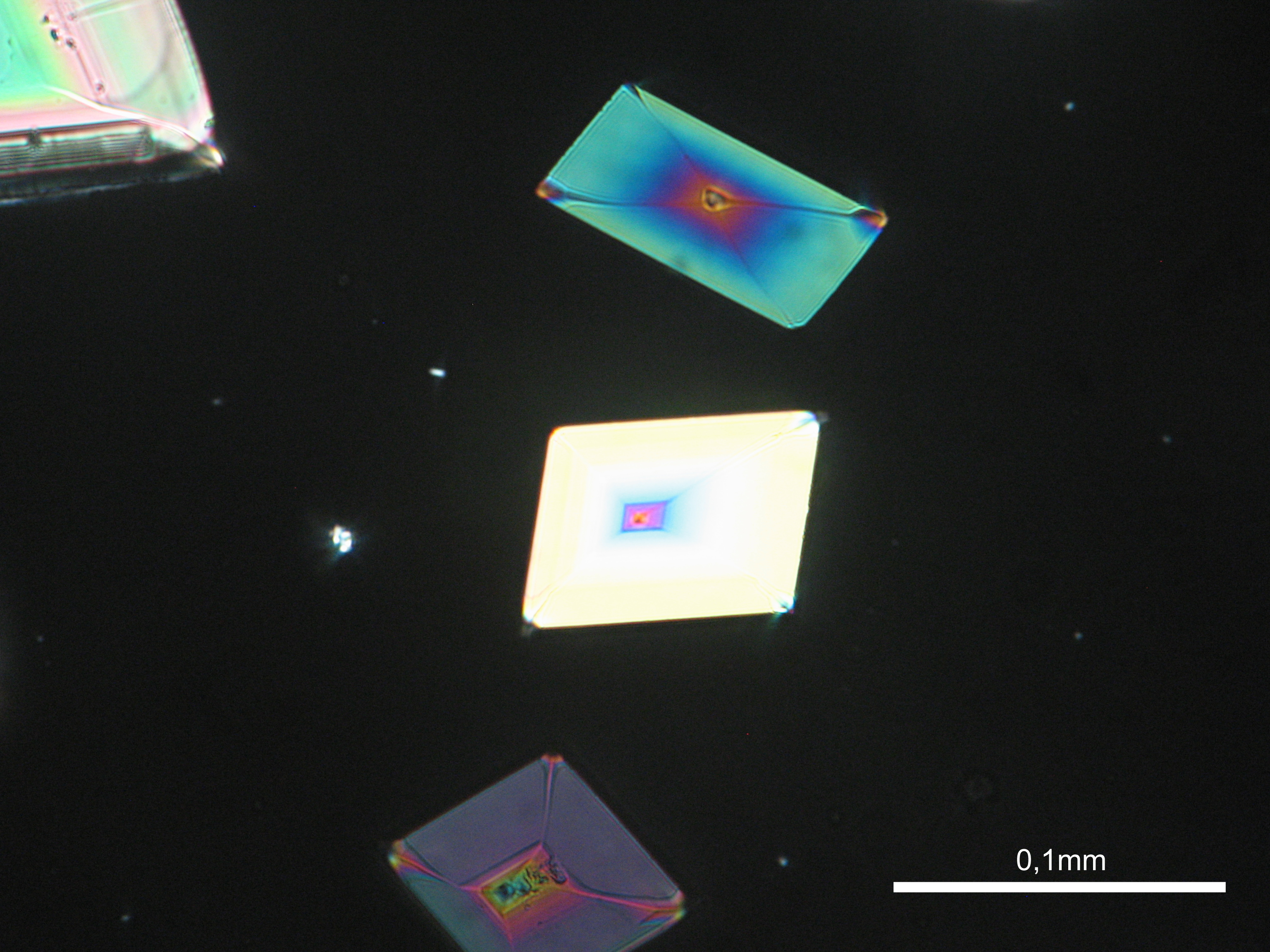
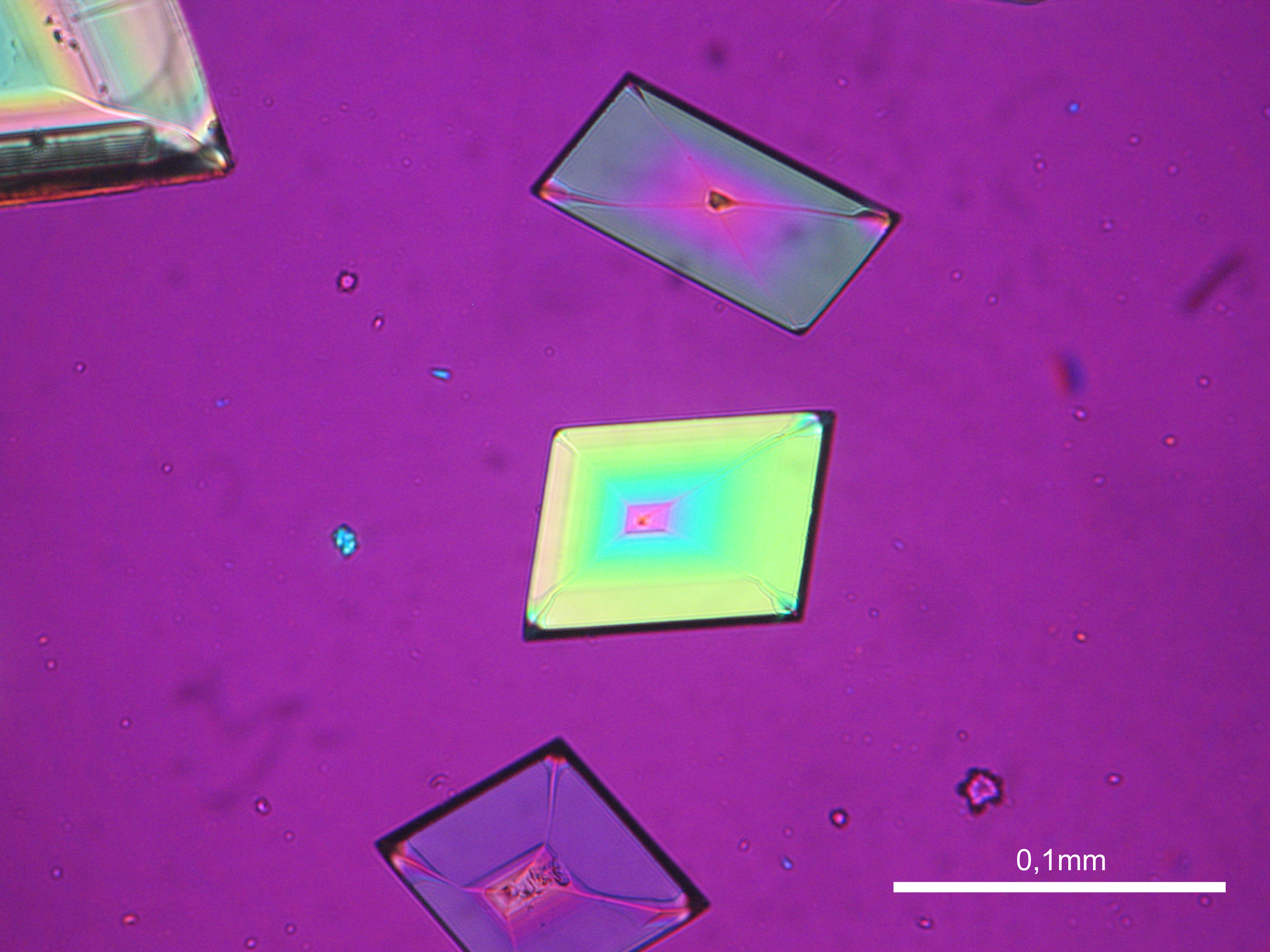
Niter[edit]
| Salt | Chemical formula | Birefringence | Refractive indices | Crystal system | Optical orientation |
|---|---|---|---|---|---|
| Niter | KNO3 | Δ = 0.171 | α = 1.335 β = 1.505 γ = 1.506 |
orthorhombic | biaxial negative |
- Niter crystallized from aqueous solution on a microscope slide
Calcium nitrate[edit]
| Salt | Chemical formula | Birefringence | Refractive indices | Crystal system | Optical orientation |
|---|---|---|---|---|---|
| Nitrocalcite |
- Calcium nitrate crystallized from aqueous solution on a microscope slide
Magnesium nitrate[edit]
| Salt | Chemical formula | Birefringence | Refractive indices | Crystal system | Optical orientation |
|---|---|---|---|---|---|
| Nitromagnesite | Mg(NO3)2•6H2O | Δ = 0.166 | nx = 1.34 ny = 1.506 nz = 1.506 |
monoclinic | negative |
- Magnesium nitrate crystallized from aqueous solution on a microscope slide
- Mg-NO3-2 (1).jpg Figure 14:Magnesium nitrate kristalle unter polarisiertem Licht mit Analysator und Rot I
Gypsum[edit]
| Salt | chemical formula | Birefringence | Refractive indices | Crystal system | Optical orientation |
|---|---|---|---|---|---|
| Gypsum | CaSO4•2H2O | Δ = 0.0092 | α = 1.5207 β = 1.5230 γ = 1.5299 |
monoclinic | biaxial positive |
- Gypsum crystallized from aqueous solution on a microscope slide
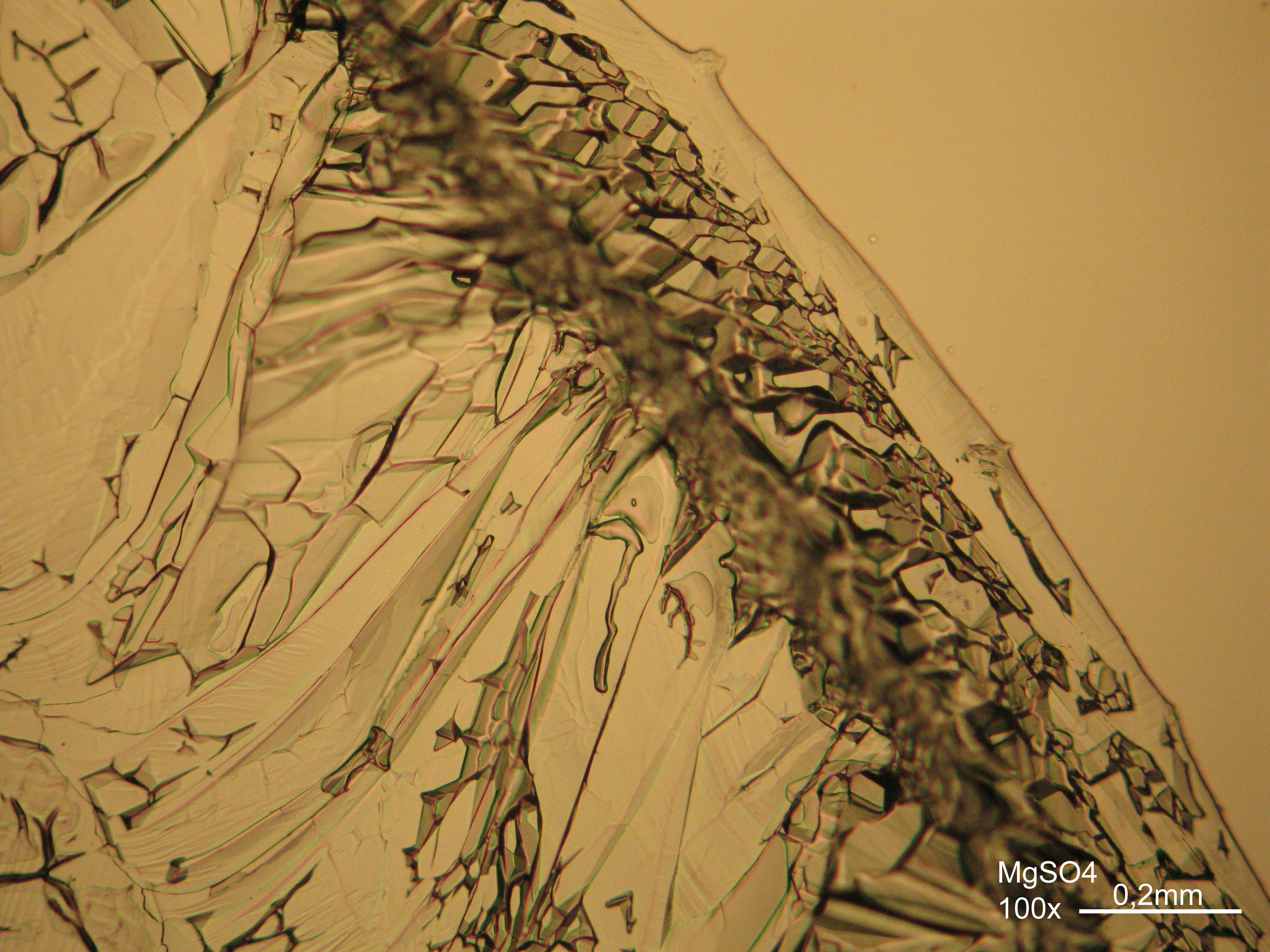
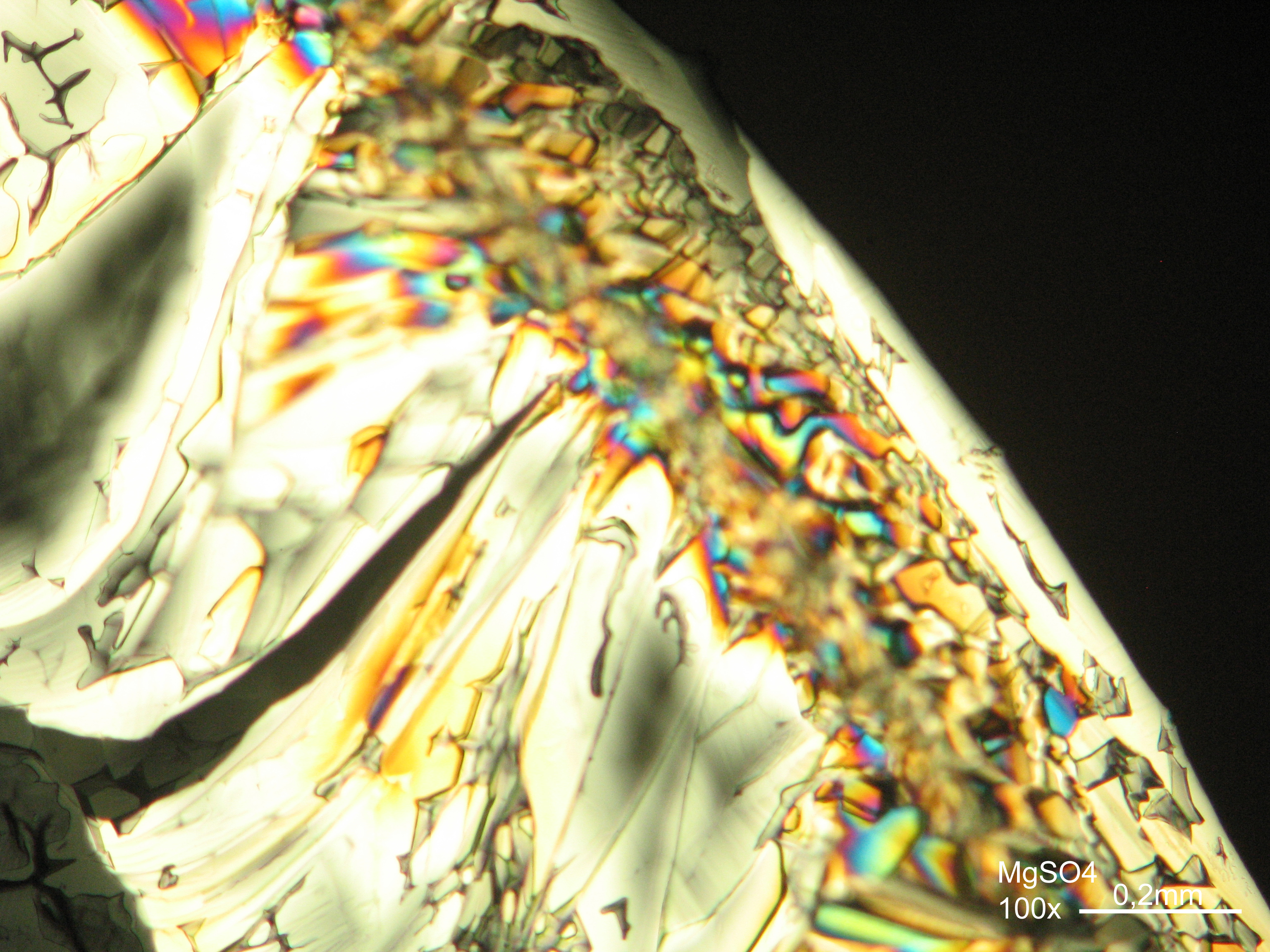
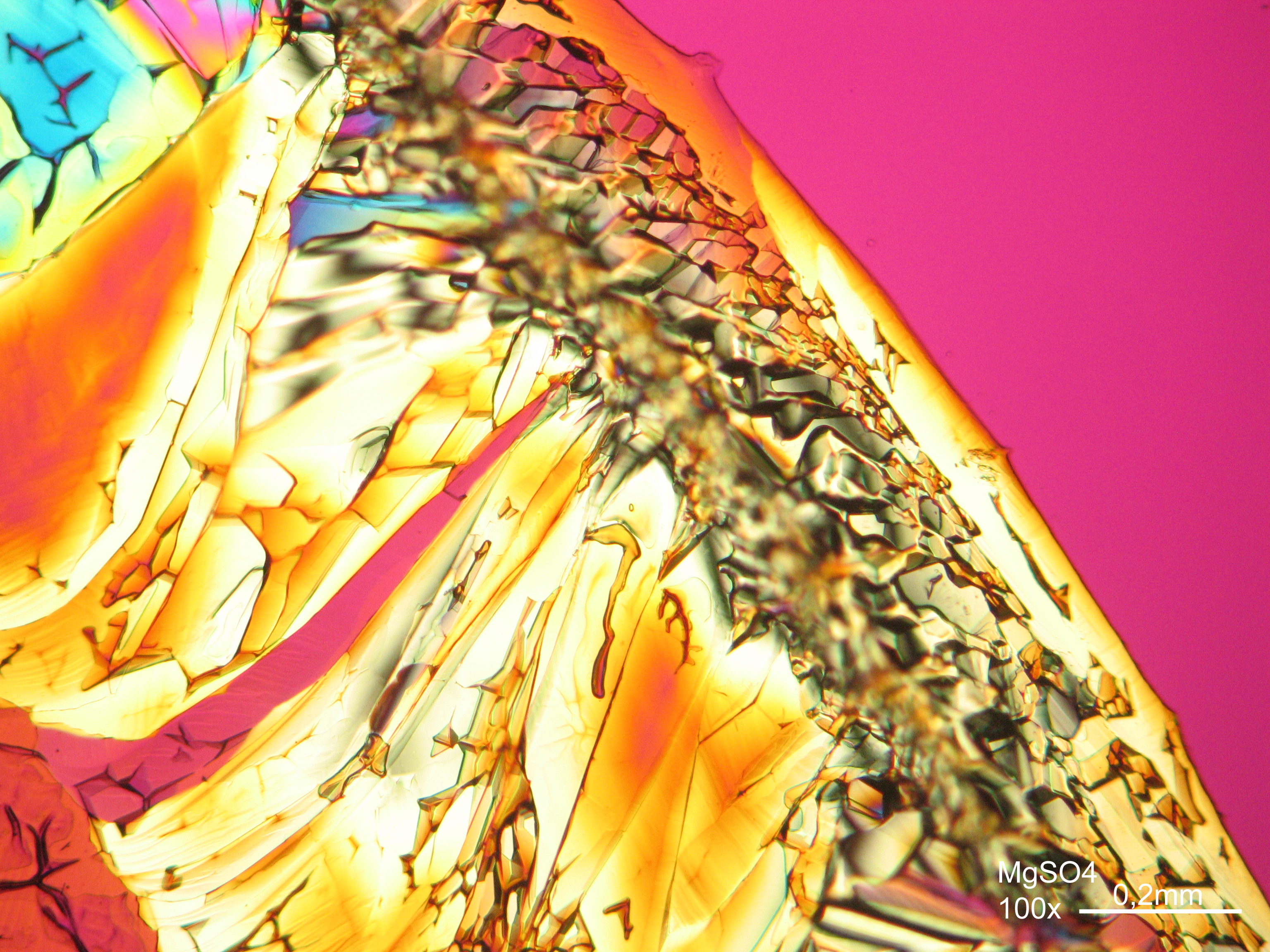
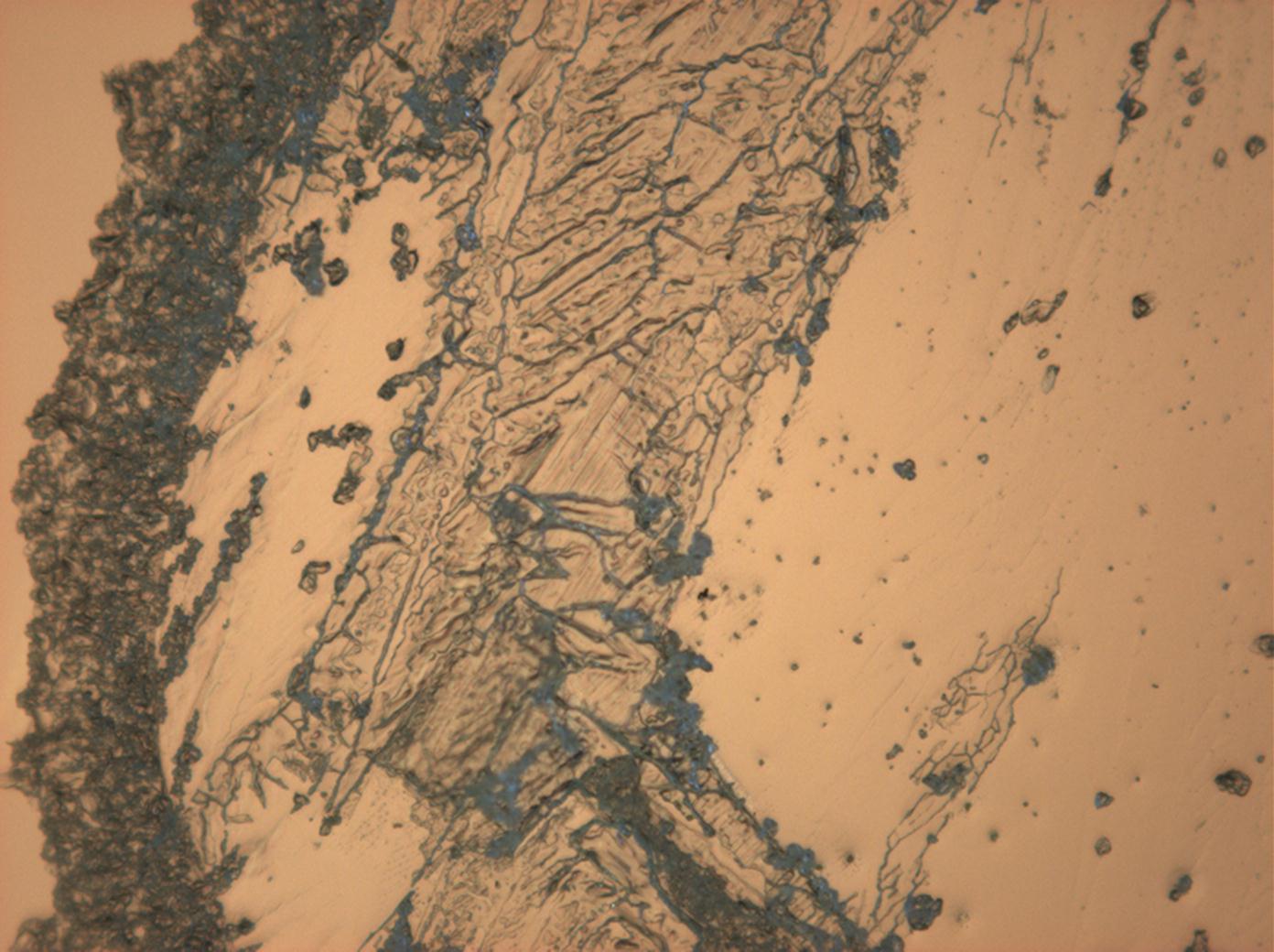
Magnesium sulfate[edit]
| Salt | chemical formula | Birefringence | Refractive indices | Crystal system | Optical orientation |
|---|---|---|---|---|---|
| Epsomite | MgSO4•7H2O | Δ = 0.0284 | nx = 1.432 ny = 1.453 nz = 1.4609 |
orthorhombic | biaxial negative |
- Magnesium sulfate crystallized from aqueous solution on a microscope slide
Sodium sulfate[edit]
| Salt | chemical formula | Birefringence | Refractive indices | Crystal system | Optical orientation |
|---|---|---|---|---|---|
| Thenardite | Na2SO4 | Δ = 0.015 | nx = 1.468 ny = 1.473 nz = 1.483 |
orthorhombic | positive |
- Sodium sulfate crystallized from aqueous solution on a microscope slide
Sodium carbonate[edit]
| Salt | chemical formula | Birefringence | Refractive indices | Crystal system | Optical orientation |
|---|---|---|---|---|---|
| Natrite | Na2CO3 | Δ = 0.131 | nx = 1.415 ny = 1.535 nz = 1.546 |
monoclinic | biaxial negative |
- Sodium carbonate crystallized from aqueous solution on a microscope slide
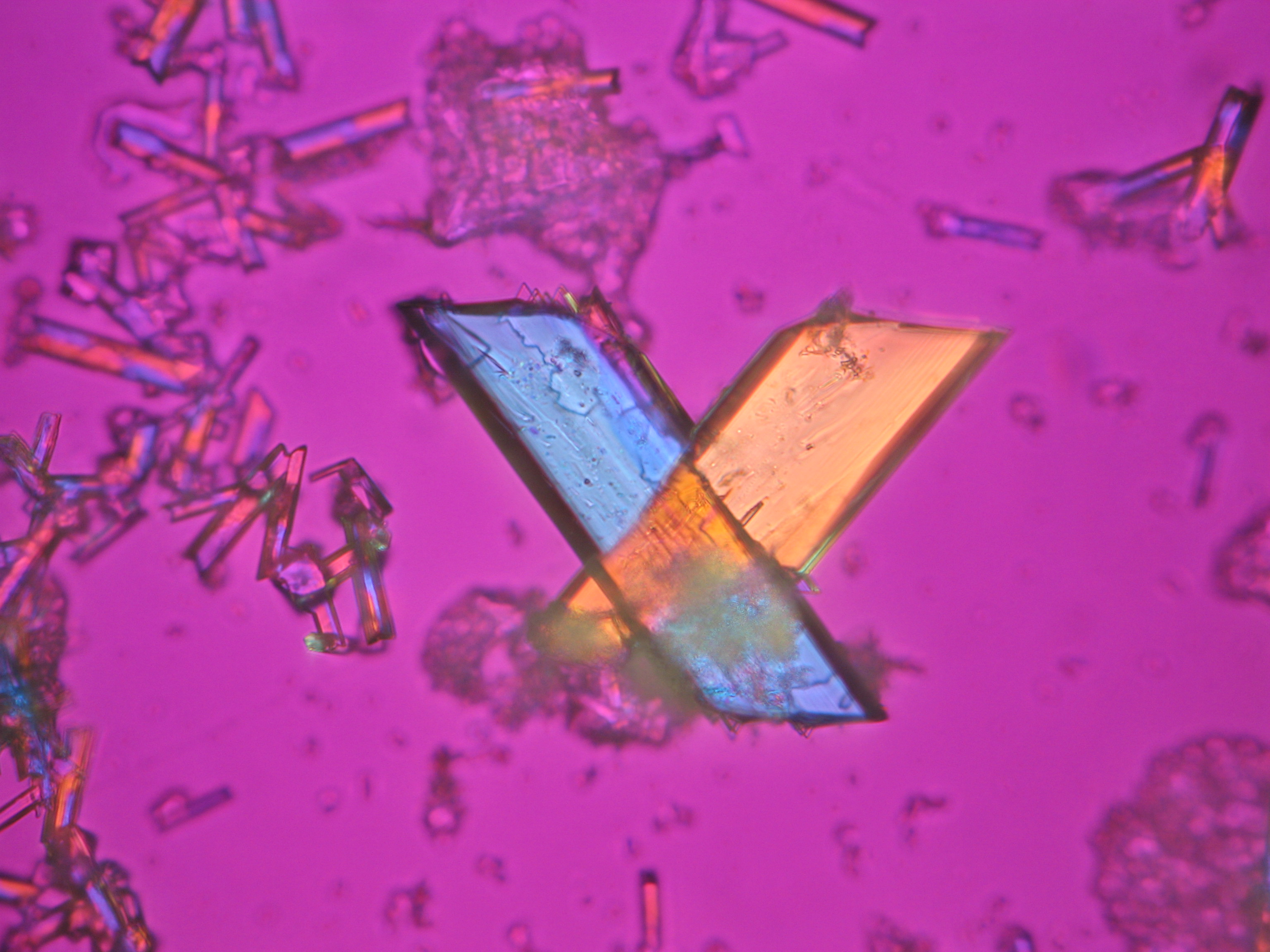
Sodium acetate[edit]
- Sodium acetate crystallized from aqueous solution