Nitrocalcite: Difference between revisions
Jump to navigation
Jump to search
Weblinks
No edit summary |
No edit summary |
||
| Line 9: | Line 9: | ||
|Crystal_System =monoclinic | |Crystal_System =monoclinic | ||
|Crystal_Structure = | |Crystal_Structure = | ||
|Deliqueszenzhumidity = 53,6 | |Deliqueszenzhumidity = 56,5% (10°C), 53,6% (20°C), 50,5% (25°C) | ||
|Solubility = | |Solubility = 2660 g/l | ||
|Density = 1,82 g/cm<sup>3</sup> | |Density = 1,82 g/cm<sup>3</sup> | ||
|MolVolume = 129,8 cm<sup>3</sup>/mol | |MolVolume = 129,8 cm<sup>3</sup>/mol | ||
Revision as of 08:40, 7 July 2011
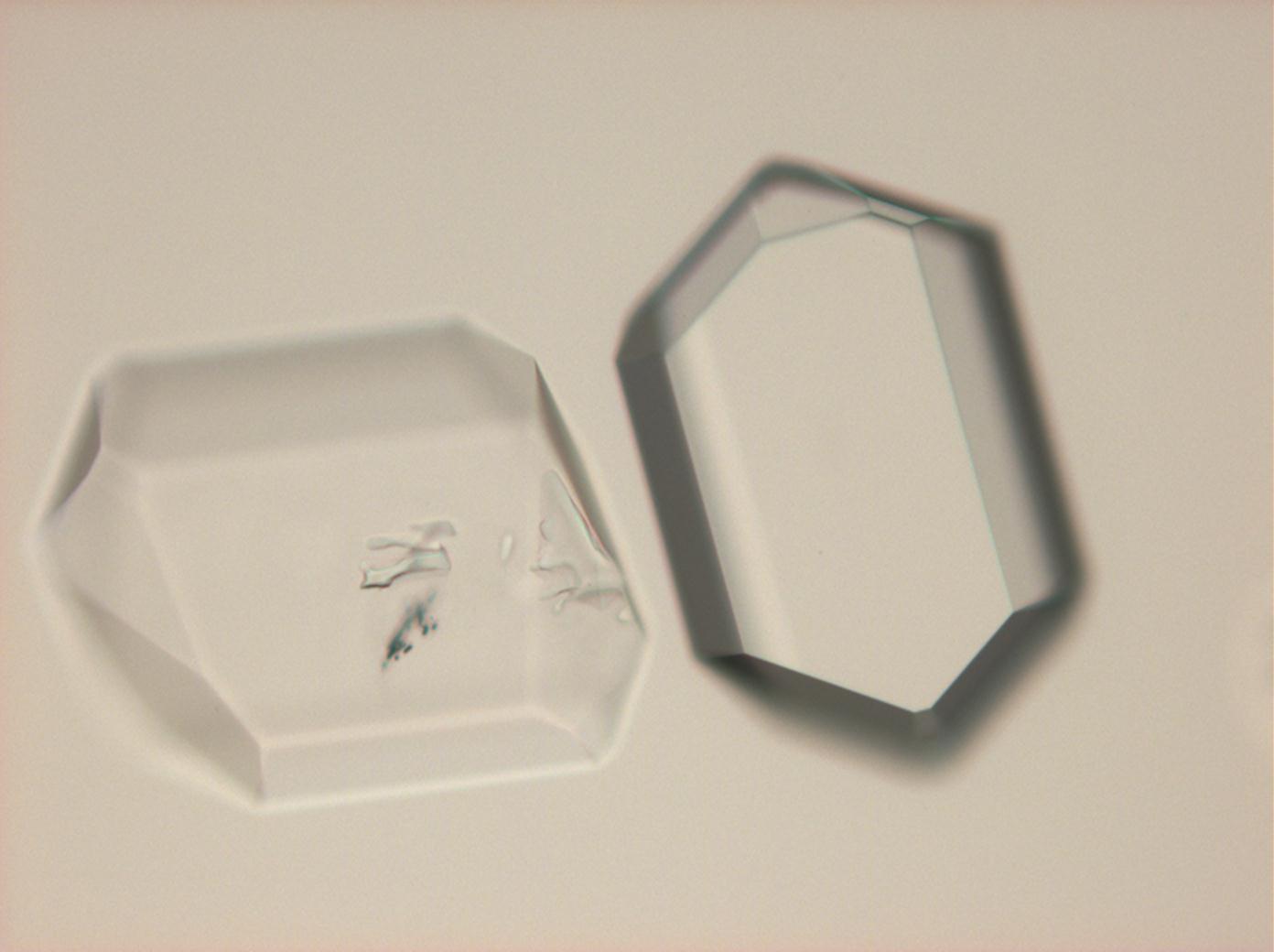
| Nitrocalcite[1] | |
| |
| Mineralogical name | Nitrocalcite |
| Chemical name | Calcium Nitrate Tetrahydrate |
| Trivial name | Nitrate of lime |
| Chemical formula | Ca(NO3)2•4H2O |
| Other forms | |
| Crystal system | monoclinic |
| Crystal structure | |
| Deliquescence humidity 20°C | 56,5% (10°C), 53,6% (20°C), 50,5% (25°C) |
| Solubility (g/l) at 20°C | 2660 g/l |
| Density (g/cm³) | 1,82 g/cm3 |
| Molar volume | 129,8 cm3/mol |
| Molar weight | 236,16 g/mol |
| Transparency | transparent |
| Cleavage | |
| Crystal habit | |
| Twinning | |
| Phase transition | |
| Chemical behavior | |
| Comments | |
| Crystal Optics | |
| Refractive Indices | nx = 1,465 ny = 1,498 nz = 1,504 |
| Birefringence | Δ = 0,039 |
| Optical Orientation | negative |
| Pleochroism | |
| Dispersion | |
| Used Literature | |
| {{{Literature}}} | |
back to Nitrate
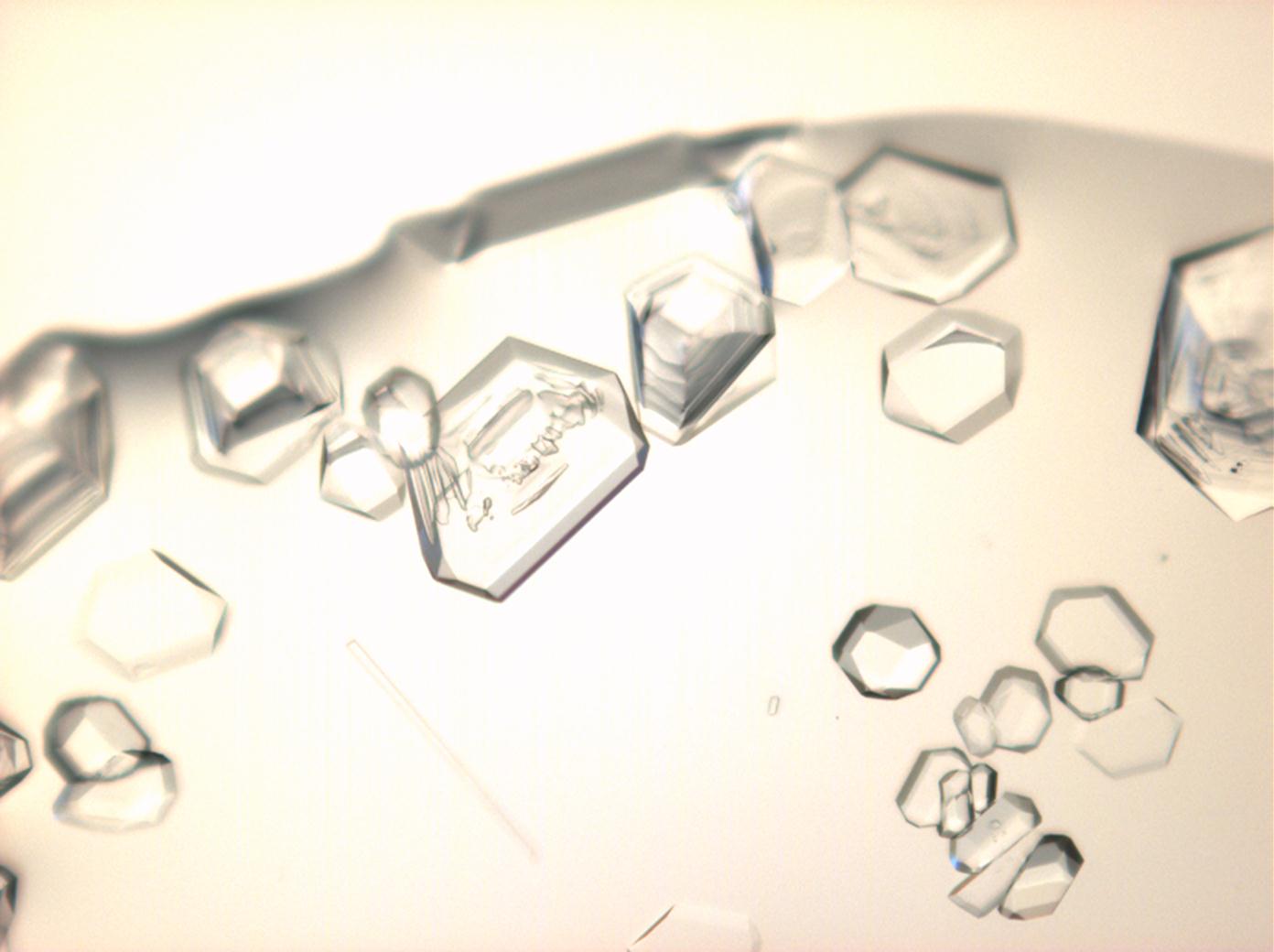
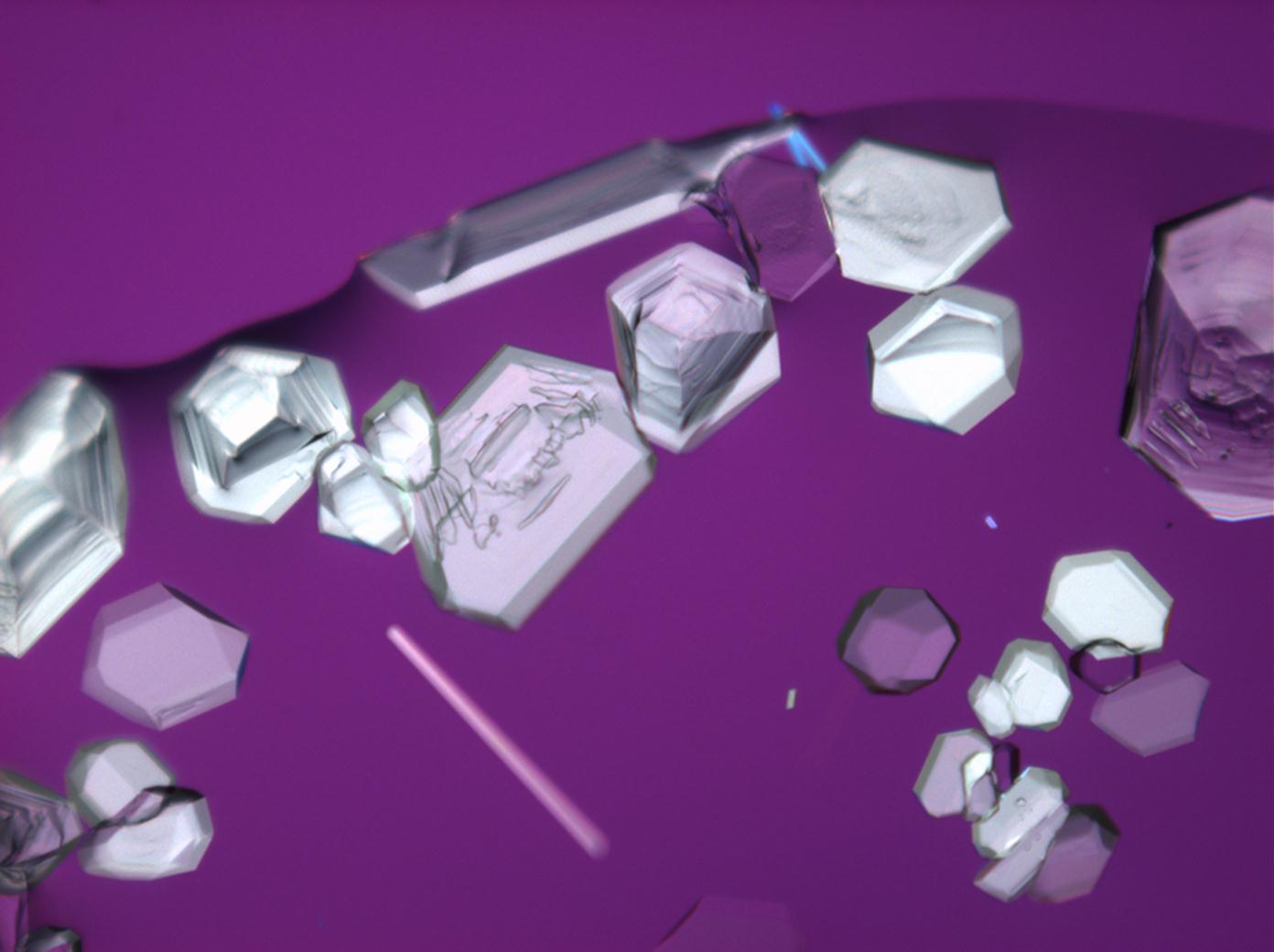
Under the polarising microscope[edit]
- Crysallised from a saturated solution with ethanol addition
- Crystallised in a micro climate chamber
Weblinks
[edit]
- ↑ http://www.mindat.org/min-2919.html seen on 29.07.2010