Nitrocalcite: Difference between revisions
Jump to navigation
Jump to search
Weblinks
No edit summary |
|||
| Line 36: | Line 36: | ||
==Under the polarising microscope == | ==Under the polarising microscope == | ||
<gallery caption=" | <gallery caption="Crystallized from a saturated solution with ethanol addition" widths="200px" heights="150px" perrow="3"> | ||
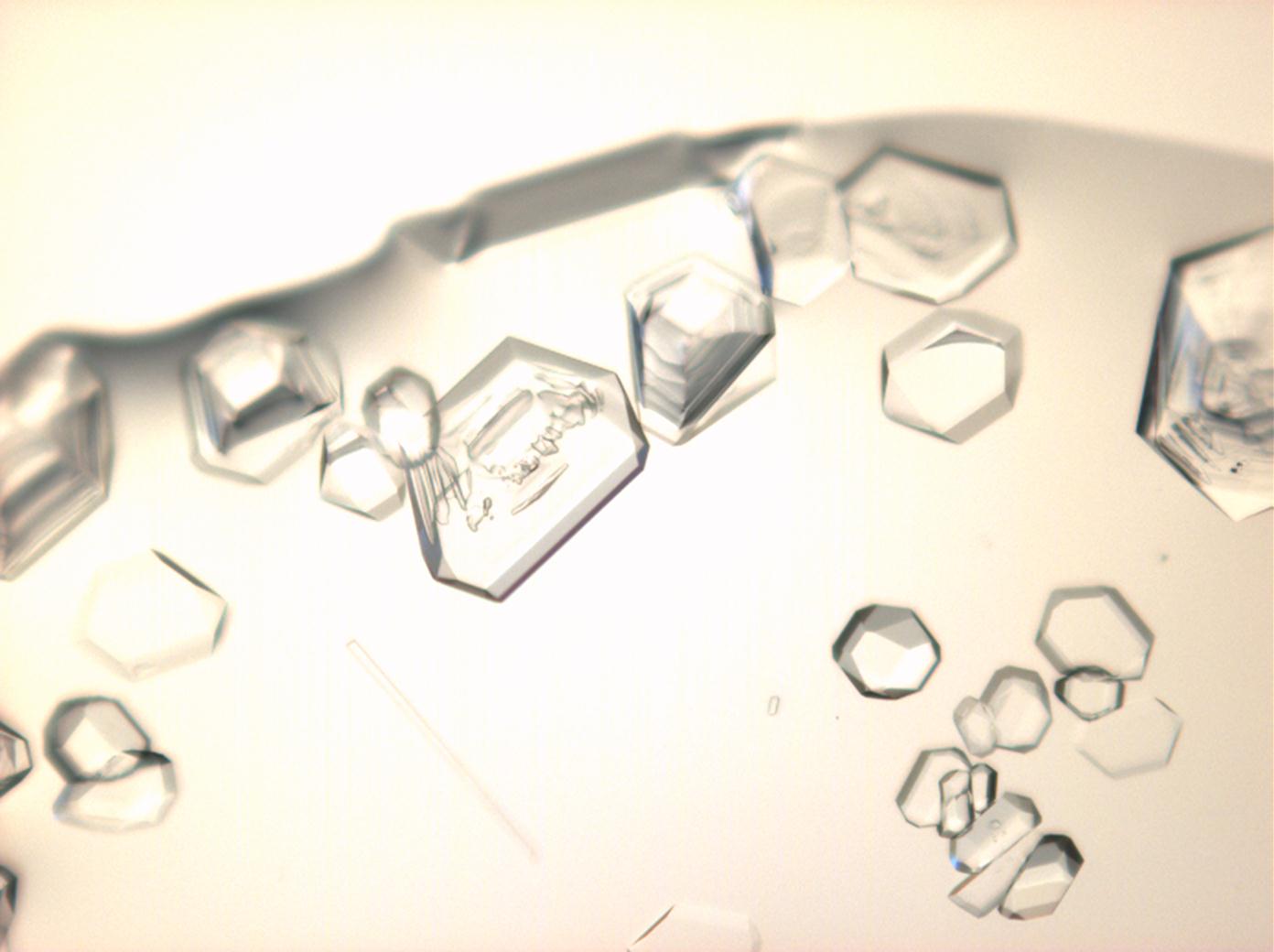
Image:HJS Ca(NO3)2 101703-4-6.jpg |in simple | Image:HJS Ca(NO3)2 101703-4-6.jpg |in simple polarized light | ||
Image:HJS Ca(NO3)2 101703-4-5.jpg|under crossed | Image:HJS Ca(NO3)2 101703-4-5.jpg|under crossed polarizers | ||
Image: HJS Ca(NO3)2 101703-4-4.jpg|under crossed | Image: HJS Ca(NO3)2 101703-4-4.jpg|under crossed polarizers and Red I | ||
</gallery> <gallery caption=" | </gallery> <gallery caption="Crystallized in a [[micro climate chamber]]" widths="200px" heights="150px" perrow="3"> | ||
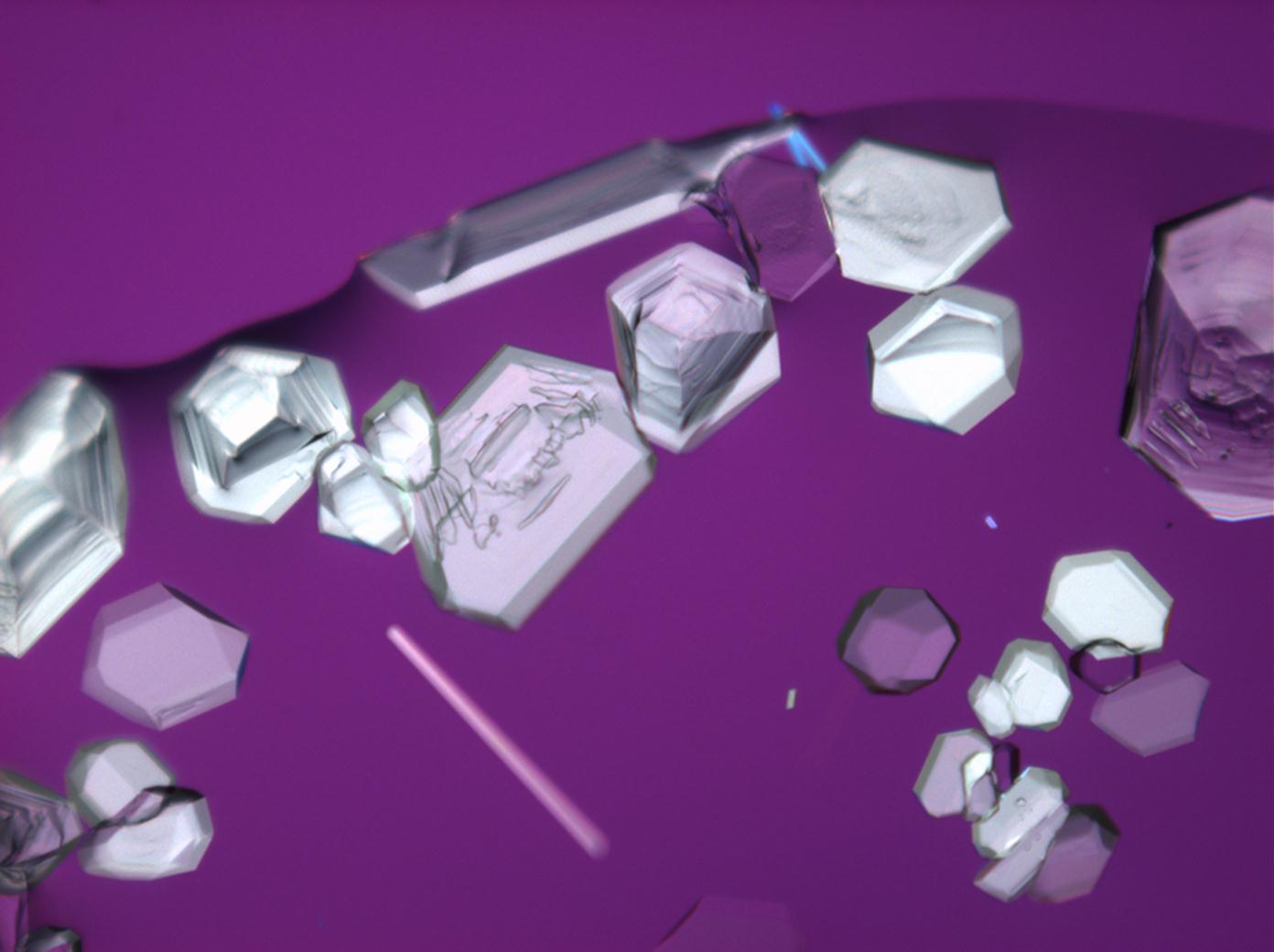
Image: HJS Ca(NO3)2 102003-4MC-0.jpg|in simple | Image: HJS Ca(NO3)2 102003-4MC-0.jpg|in simple polarized light | ||
Image: HJS Ca(NO3)2 102003-4MC-1.jpg|under crossed | Image: HJS Ca(NO3)2 102003-4MC-1.jpg|under crossed polarizers and Red I | ||
</gallery> | </gallery> | ||
== Weblinks<br> == | == Weblinks<br> == | ||
Revision as of 15:03, 28 January 2015
| Nitrocalcite[1] | |
| |
| Mineralogical name | Nitrocalcite |
| Chemical name | Calcium Nitrate Tetrahydrate |
| Trivial name | Nitrate of lime |
| Chemical formula | Ca(NO3)2•4H2O |
| Other forms | |
| Crystal system | monoclinic |
| Crystal structure | |
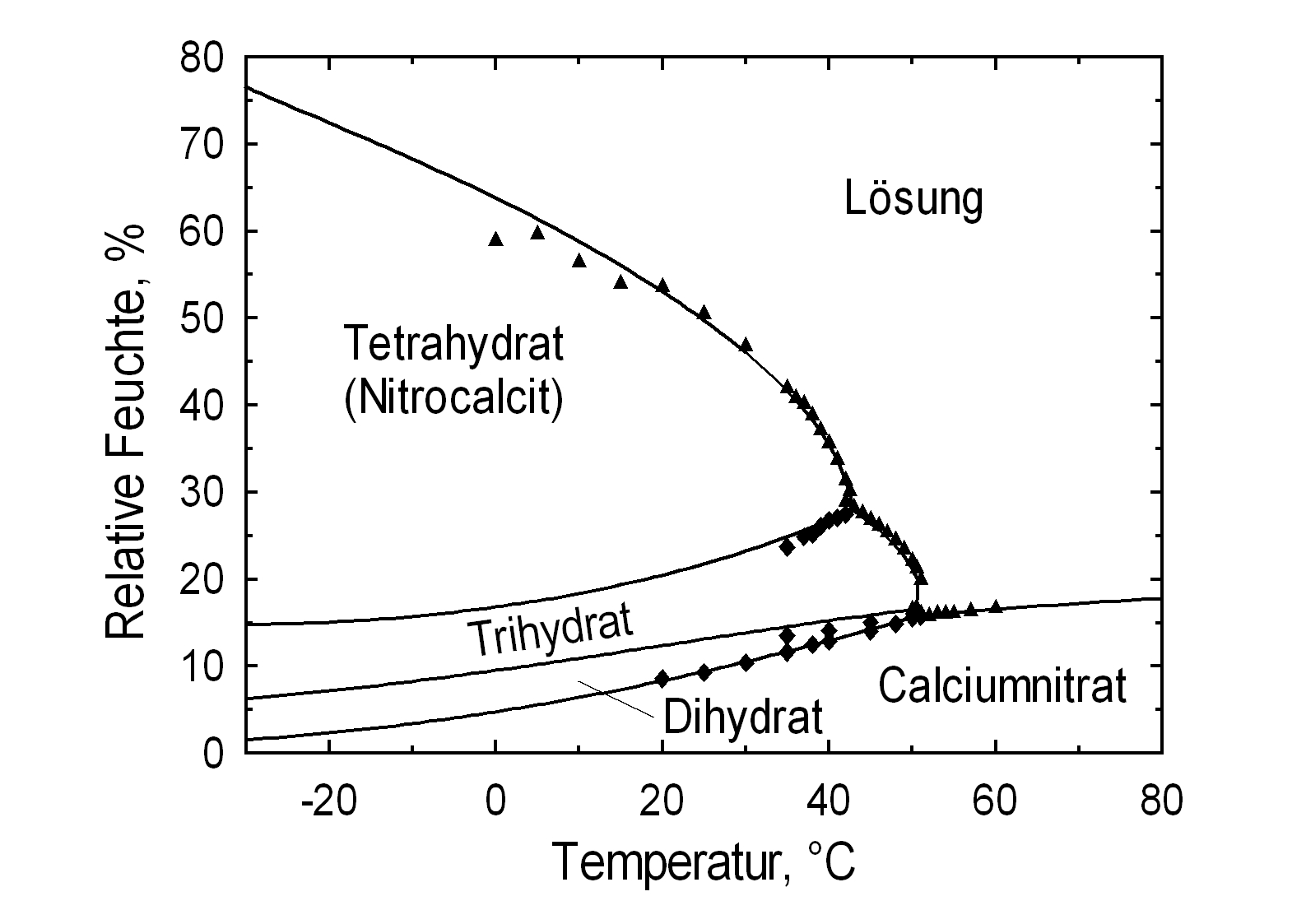
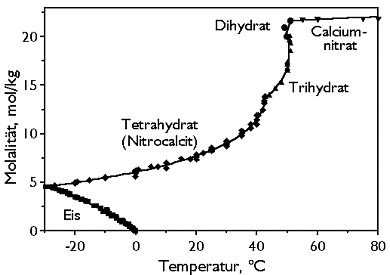
| Deliquescence humidity 20°C | 56.5% (10°C), 53.6% (20°C), 50.5% (25°C) |
| Solubility (g/l) at 20°C | 2660 g/l |
| Density (g/cm³) | 1.82 g/cm3 |
| Molar volume | 129.8 cm3/mol |
| Molar weight | 236.16 g/mol |
| Transparency | transparent |
| Cleavage | |
| Crystal habit | |
| Twinning | |
| Phase transition | |
| Chemical behavior | |
| Comments | |
| Crystal Optics | |
| Refractive Indices | nx = 1.465 ny = 1.498 nz = 1.504 |
| Birefringence | Δ = 0.039 |
| Optical Orientation | negative |
| Pleochroism | |
| Dispersion | |
| Used Literature | |
| {{{Literature}}} | |
back to Nitrate
Phase Diagrams[edit]
Under the polarising microscope[edit]
- Crystallized from a saturated solution with ethanol addition
- Crystallized in a micro climate chamber
Weblinks
[edit]
- ↑ http://www.mindat.org/min-2919.html seen on 29.07.2010