Sodium sulfate: Difference between revisions
No edit summary |
No edit summary |
||
| Line 3: | Line 3: | ||
back to [[Sulfate]] | back to [[Sulfate]] | ||
{{UnderConstruction}} | |||
{{ | |||
==Abstract== | ==Abstract== | ||
| Line 17: | Line 16: | ||
[[Sodium sulfate heptahydrate]] Na<sub>2</sub>SO<sub>4</sub>•7H<sub>2</sub>O metastable <br> | [[Sodium sulfate heptahydrate]] Na<sub>2</sub>SO<sub>4</sub>•7H<sub>2</sub>O metastable <br> | ||
[[Mirabilite]] Na<sub>2</sub>SO<sub>4</sub>•10H<sub>2</sub>O | [[Mirabilite]] Na<sub>2</sub>SO<sub>4</sub>•10H<sub>2</sub>O | ||
== Occurrence == | |||
Both [[thenardite]] and [[mirabilite]] occur as natural minerals. In nature sodium sulfate occurs in mineral waters in the form of double salts, as deposits of former salt lakes. The hydrated sodium sulfate was first described by Glauber in 1658 where he called it "sal mirabilis". [[Mirabilite]] is also known as "Glauber's salt" in honor of its discoverer. | |||
== Origin and formation of thenardite / mirabilite in monuments == | |||
When sodium ions in conjunction with other anions enter porous inorganic building materials, sodium sulfate may be formed by reaction with sulfate contributed by other sources, for example air contaminated with sulfur oxide gases. Portland cement contains a certain amount of sodium or potassium sulfate. In Germany, the standardization institute (DIN) allows a content of up to 0.5 % soluble alkalis. This means that 100 kg of Portland cement containing only 0.1% soluble Na<sub>2</sub>O can form 520g of [[Mirabilite]] when reacting with sulfate [calculation by Arnold/Zehnder 1991]. Sodium ions can also enter into monuments from various cleaning materials and, in older restoration products, such as water glass. Ground water, and even surface water, are also a possible source of Na<sup>+</sup>-ions as well as sulfate ions. De-icing road salt may contain a large amount of soluble [[Halite|sodium chloride]]. Finally, in coastal areas, sea water is a significant source of [[Halite|NaCl]]. | |||
==Solubility== | ==Solubility== | ||
| Line 25: | Line 34: | ||
The phases of sodium sulfate are easily soluble in water (figure 1), so they have a high mobility in porous materials as well. The solubility of the phases depends on the temperature.<br> | The phases of sodium sulfate are easily soluble in water (figure 1), so they have a high mobility in porous materials as well. The solubility of the phases depends on the temperature.<br> | ||
The decahydrate [[mirabilite]] is stable below 32.4 °C. Above this temperature the anhydrous [[thenardite]] is the stable crystalline phase, which in turn is metastable below this transition temperature. In the case of a temperature | The decahydrate [[mirabilite]] is stable below 32.4 °C. Above this temperature the anhydrous [[thenardite]] is the stable crystalline phase, which in turn is metastable below this transition temperature. In the case of a temperature drop in a solution that is saturated with respect to thenardite, high supersaturations with respect to mirabilite and with that its precipitation are possible, which involves a certain damage potential. | ||
<br clear="all"> | <br clear="all"> | ||
| Line 47: | Line 56: | ||
|} | |} | ||
<br clear="all"> | <br clear="all"> | ||
<!-- | |||
'''Water vapor sorption: ''' | |||
[[file:Deliqueszenz Mirabilit, Thenardit .JPG|thumb|350px|right|'''Figure 3''': Deliquescence points of pure salts thenardite and mirabilite <bib id="Arnold.etal:1991"/>]] | |||
The table below shows additional information for estimating the hygroscopicity of sodium sulfate for the sorption behavior of pure salt and the mixture with [[Halite|halite]] at different relative humidity levels: | |||
<br> | |||
{|border="2" cellspacing="0" cellpadding="4" width="52%" align="center" class="wikitable" | |||
|+''Table 2''': Moist sorption of sodium sulphate in M.% after 56 days of storage [after <bib id="Vogt.etal:1993"/>]'' | |||
|- | |||
|bgcolor = "#F0F0F0" | '''Air humidity''' | |||
|bgcolor = "#F0F0F0" align="center"| '''87% r.F.''' | |||
|bgcolor = "#F0F0F0" align="center"| '''81% r.F.''' | |||
|bgcolor = "#F0F0F0" align="center"| '''79% r.F.''' | |||
|- | |||
|bgcolor = "#F7F7F7" | '''Na<sub>2</sub>SO<sub>4</sub>''' | |||
|bgcolor = "#FFFFEO" align="center"| 79 | |||
|bgcolor = "#FFFFEO" align="center"| 0 | |||
|bgcolor = "#FFFFEO" align="center"| 0 | |||
|- | |||
|bgcolor = "#F7F7F7" | '''Na<sub>2</sub>SO<sub>4</sub>+NaCl''' (1:1 molar mixture) | |||
|bgcolor = "#FFFFEO" align="center"| 157 | |||
|bgcolor = "#FFFFEO" align="center"| 32 | |||
|bgcolor = "#FFFFEO" align="center"| 15 | |||
|} | |||
--> | |||
==Hygroscopicity== | |||
The deliquescence behaviour of the different sodium sulfate phases is shown in figure 2 as a function of temperature. The equilibrium humidities of the [[thenardite]]/[[mirabilite]] conversion are also represented.<br> | |||
The temperature dependencce for thenardite and for mirabilite are contradictory, as the deliquescence humidity of mirabilite decreases with increasing temperature whereas those of thenardite increases and it is influenced to a lesser extend by temperature changes. | |||
<br clear="all"> | |||
[[File:D Na2SO4e.jpg|thumb|800px|right|'''Figure 2:''' Deliquescnece behaviour in the system Na<sub>2</sub>SO<sub>4</sub>-H<sub>2</sub>O as a funcction of temperature. The water activity ''a<sub>w</sub>'' is plotted versus the temperature. Deliquescence humidities of the different phases are labled by different colours. Dashed lines indicate metastable equilibria. Equilibrium humidities for the thenaridte/mirabilite converison are also shown. according to <bib id="Steiger.etal:2008"/>.]] | |||
<br clear="all"> | |||
<br clear="all"> | |||
{|border="2" cellspacing="0" cellpadding="4" width="35%" align="left" class="wikitable" | |||
|+''Table 2: Deliquescence and equilibrium humidities at 20°C [according to <bib id="Steiger.etal:2008"/>].'' | |||
|- | |||
|bgcolor = "#F0F0F0" align=center| '''considered phase transition''' | |||
|bgcolor = "#F0F0F0" align=center| '''deliquescence or equilibrium humidity at 20 °C''' | |||
|- | |||
|bgcolor = "#FFFFEO" align=center| [[sodium sulfate phase III]]-solution | |||
|bgcolor = "#FFFFEO" align=center| 82.9 % | |||
|- | |||
|bgcolor = "#FFFFEO" align=center| [[thenardite]]-solution | |||
|bgcolor = "#FFFFEO" align=center| 86.6 % | |||
|- | |||
|bgcolor = "#FFFFEO" align=center| [[sodium sulfate heptahydrate]]-solution | |||
|bgcolor = "#FFFFEO" align=center| 89.1 % | |||
|- | |||
|bgcolor = "#FFFFEO" align=center| [[mirabilite]]-solution | |||
|bgcolor = "#FFFFEO" align=center| 95.6 % | |||
|- | |||
|bgcolor = "#FFFFEO" align=center| [[thenardite]]-[[mirabilite]] | |||
|bgcolor = "#FFFFEO" align=center| 76.4 % | |||
|} | |||
<br clear="all"> | |||
== Hydration behavior == | |||
[[file:Mirabilit Thenardit.ogg|thumb|400px|right|Conversion of mirabilite (?) into thenardite]] | |||
The Na<sub>2</sub>SO<sub>4</sub> – H<sub>2</sub>O system: | |||
The only stable forms of sodium sulfate are the decahydrate ([[Mirabilite|mirabilite]]) and the anhydrite (thenardite). The generation of mirabilite by recrystallization of the salt from an aqueous supersaturated solution occurs at 32.4°C. In particular, the transition from thenardite to mirabilite and the incorporation of 10 water molecules in the crystal lattice causes a volume expansion of 320%. This transition happens at a relatively low temperature (32-35°C), the damage caused by this salt is highly dependent on the temperature and thus on the environment. This temperature range is given as a guide, because this transition could happen for example at 25°C at 80% relative humidity, or even at 0°C at 60.7% relative humidity [information from Gmelin]. Due to this strong dependence on the environmental parameters, an estimate of the damage caused on buildings by crystallization and hydration of sodium sulfate are very difficult to obtain. | |||
<!-- | |||
== Hydration pressure == | |||
The hydration pressure, which occurs at the transition from thenardite to [[mirabilite]], is highly dependent on the existing relative humidity and temperature conditions. This correlation is shown in table 3 as follows: | |||
<br clear="all"> | |||
{|border="2" cellspacing="0" cellpadding="4" width="52%" align="left" class="wikitable" | |||
|+''Table 3''': Hydration pressure thenardite-[[mirabilite]] nach <bib id="Winkler.etal:1970"/>'' | |||
|- | |||
|bgcolor = "#F0F0F0" align=center| '''RH %''' | |||
|bgcolor = "#F0F0F0" align=center| '''20.0 °C''' | |||
|bgcolor = "#F0F0F0" align=center| '''25.0 °C''' | |||
|bgcolor = "#F0F0F0" align=center| '''30.0 °C''' | |||
|- | |||
|bgcolor = "#F7F7F7" align=center| '''100''' | |||
|bgcolor = "#FFFFEO" align="center"| 48.9 N/mm<sup>2</sup> | |||
|bgcolor = "#FFFFEO" align="center"| 40.5 N/mm<sup>2</sup> | |||
|bgcolor = "#FFFFEO" align="center"| 28.9 N/mm<sup>2</sup> | |||
|- | |||
|bgcolor = "#F7F7F7" align=center| '''95,0''' | |||
|bgcolor = "#FFFFEO" align="center"| 41.3 N/mm<sup>2</sup> | |||
|bgcolor = "#FFFFEO" align="center"| 32.7 N/mm<sup>2</sup> | |||
|bgcolor = "#FFFFEO" align="center"| 23.3 N/mm<sup>2</sup> | |||
|- | |||
|bgcolor = "#F7F7F7" align="center"| '''90,0''' | |||
|bgcolor = "#FFFFEO" align="center"| 33.5 N/mm<sup>2</sup> | |||
|bgcolor = "#FFFFEO" align="center"| 24.9 N/mm<sup>2</sup> | |||
|bgcolor = "#FFFFEO" align="center"| 13.7 N/mm<sup>2</sup> | |||
|- | |||
|bgcolor = "#F7F7F7" align="center"| '''85,0''' | |||
|bgcolor = "#FFFFEO" align="center"| 25.5 N/mm<sup>2</sup> | |||
|bgcolor = "#FFFFEO" align="center"| 16.0 N/mm<sup>2</sup> | |||
|bgcolor = "#FFFFEO" align="center"| 5.1 N/mm<sup>2</sup> | |||
|- | |||
|bgcolor = "#F7F7F7" align="center"| '''80,0''' | |||
|bgcolor = "#FFFFEO" align="center"| 16.4 N/mm<sup>2</sup> | |||
|bgcolor = "#FFFFEO" align="center"| 7.8 N/mm<sup>2</sup> | |||
|bgcolor = "#FFFFEO" align="center"| 0.0 | |||
|- | |||
|bgcolor = "#F7F7F7" align="center"| '''75.0''' | |||
|bgcolor = "#FFFFEO" align="center"| 6.7 N/mm<sup>2</sup> | |||
|bgcolor = "#FFFFEO" align="center"| 0.0 | |||
|bgcolor = "#FFFFEO" align="center"| - | |||
|} | |||
<br clear=all> | |||
The change in volume, taking place during phase transition, is to be specified at approx. 320%. | |||
<bib id="Sperling.etal:1980"/>. | |||
--> | |||
<!-- | |||
== Analytical detection == | |||
=== Microscopy<br> === | |||
'''Laboratory investigation:'''<br> Through microscopic observations regarding the solubility behavior, good solubility in water and no solubility in ethanol can be confirmed. Thenardite and mirabilite do not have morphological characteristics that could help identification with the use of simple re-crystallization experiments. | |||
<br> | |||
'''Refractive indices:''' n<sub>x</sub> = 1.468; n<sub>y</sub> =1,473; n<sub>z</sub> =1.483<br> | |||
'''Birefringence''': Δ = 0.015<br> | |||
'''Crystal class''': orthorhombic<br> | |||
<br> | |||
'''[[Polarized light microscopy]] examination:'''<br> | |||
The raw sampling material and the re-crystallized preparation change their water content, depending on the conditions of relative humidity and temperature. | |||
In dry air conditions (RH < 80% and room temperature) [[mirabilite]] looses its chemically bound water and changes to thenardite. This process can be clearly understood and reproduced using a microscope, when the process of re-crystallization is observed. Mirabillite does show the characteristic abnormal interference colors. During the moisture loss and the formation of thenardite these abnormal interference colors become weaker.<br><br>The refractive index assignment of thenardite is carried out using the immersion method. Due to the low maximum birefringence, thenardite mostly displays gray interference colors. The extinction is parallel or symmetrical. | |||
<br>'''Possible mistakes:''' | |||
Generally, the differentiation between certain kinds of sulfates (they are listed below, thenardite included) is a problem without micro-chemical determination of the anions, because the refractive indices of the salts lie very closely together and all salts show a low birefringence. It is best to use an immersion medium with a n<sub>D</sub>- value of 1,48, thus making the differentiation within this group possible. Moreover, the properties mentioned below can be consulted as criteria for determination. | |||
Thenardite is unambiguously, but indirectly determined by re-crystallizing a sample and observing the abnormal interference colors, which occur when mirabiltite is identified in its high hydrate form. | |||
{|border="2" cellspacing="0" cellpadding="4" width="100%" align="left" class="wikitable" | |||
|+''Table 3''': Characteristics for differentiating thenardite from other sulfates'' | |||
|- | |||
|bgcolor = "#F0F0F0"| '''Salt phase''' | |||
|bgcolor = "#F0F0F0"| '''Characteristic''' | |||
|- | |||
|bgcolor = "#F7F7F7"| '''[[Boussingaultite]]''' (NH<sub>4</sub>)<sub>2</sub>Mg(SO)<sub>4</sub> • 6H<sub>2</sub>0 | |||
|bgcolor = "#FFFFEO"| no abnormal interference colors/ inclined extinction | |||
|- | |||
|bgcolor = "#F7F7F7"| '''[[Schönite|Pikromerite]]''' K<sub>2</sub>Mg(SO<sub>4</sub>)<sub>2</sub> • 6H<sub>2</sub>0 | |||
|bgcolor = "#FFFFEO"| no abnormal interference colors/ inclined extinction | |||
|- | |||
|bgcolor = "#F7F7F7"|'''[[Astrakanite|Bloedite]]''' Na<sub>2</sub>Mg(SO<sub>4</sub>)<sub>2</sub> • 6H<sub>2</sub>0 | |||
|bgcolor = "#FFFFEO"| all indices >1.48 / no abnormal interference colors/ inclined extinction / optically negative orientation. | |||
|- | |||
|bgcolor = "#F7F7F7"| '''[[Aphthitalite|Glaserite]]''' K<sub>3</sub>Na(SO<sub>4</sub>)<sub>2</sub> | |||
|bgcolor = "#FFFFEO"| all indizes >1.48 / no abnormal interference colors/ inclined extinction | |||
|- | |||
|bgcolor = "#F7F7F7"| '''[[Arcanite]]''' K<sub>2</sub>SO<sub>4</sub> | |||
|bgcolor = "#FFFFEO"| all indices >1.48 / no abnormal interference colors | |||
|- | |||
|bgcolor = "#F7F7F7"| '''[[Magnesium formiate]]''' Mg(HCO<sub>2</sub>)<sub>2</sub> • 2H<sub>2</sub>O | |||
|bgcolor = "#FFFFEO"| comparatively high birefringence / no abnormal interference colors/ inclined extinction | |||
|} | |||
<br> | |||
'''Observation of mixed systems:''' | |||
The mixed system Na<sup>+</sup>– Ca<sup>2+</sup>– SO<sub>4</sub> <sup>2-</sup>: The precipitation of [[gypsum]] takes place first during re-crystallization, which is due to its low solubility. The distinct needle like habit of single gypsum crystals and aggregates remains. | |||
The precipitation of sodium sulfate takes place later. The actual crystal growth takes place much faster. The morphology is non-specific. | |||
Mixed system Na<sup>+</sup>– SO<sub>4</sub> <sup>2-</sup>– Cl<sup>-</sup>: The precipitation of both kinds of particle starts approximately at the same time, [[halite]] with its characteristic morphology, [[sodium sulfate]] in extremely varying forms. | |||
<!-- | |||
=== X-ray diffractometry === | |||
=== Raman-Spectroscopy === | |||
=== DTA / TG === | |||
=== IR-Spectroscopy === | |||
<br> | |||
<br> | |||
<br> | |||
--> | |||
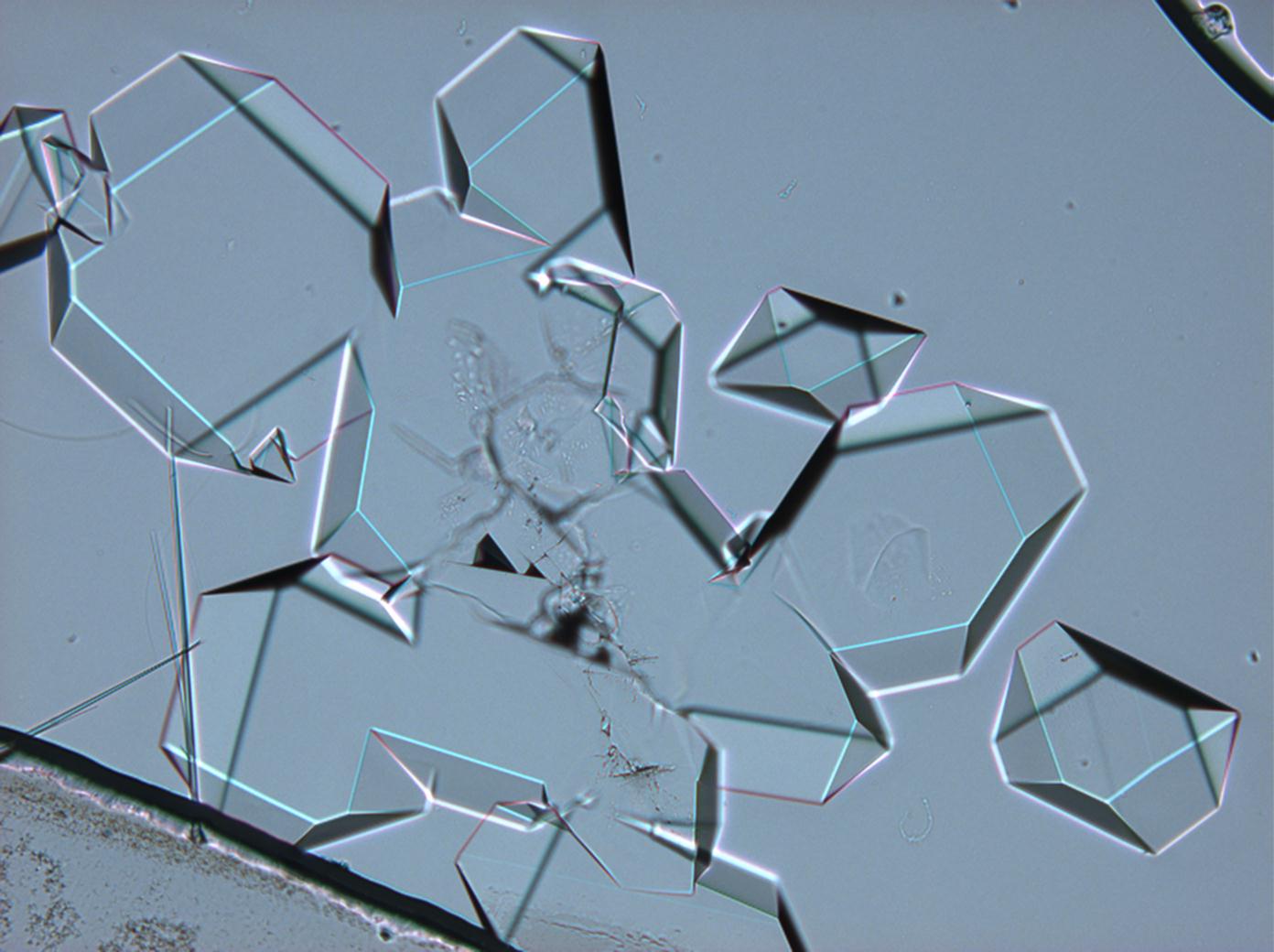
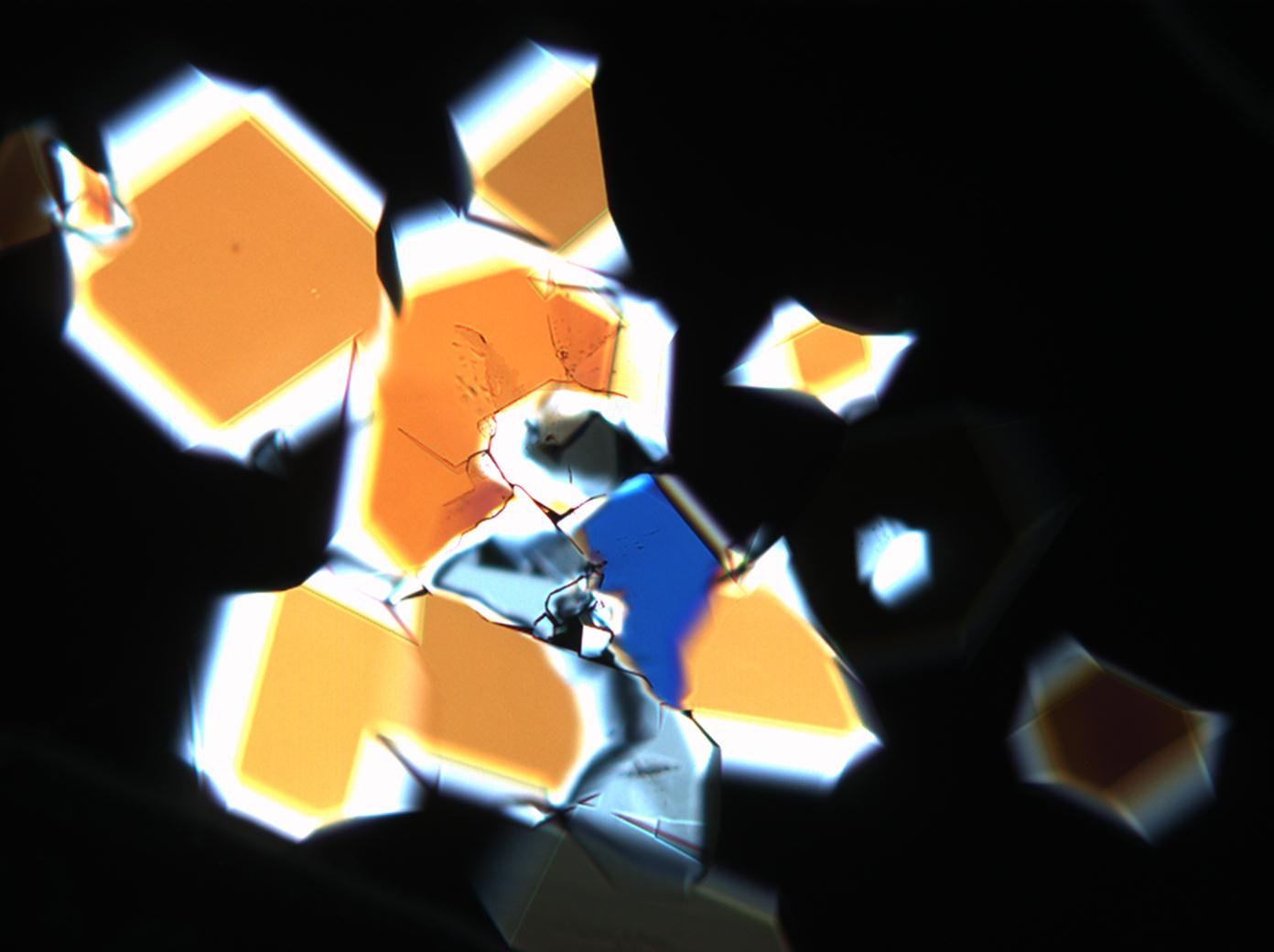
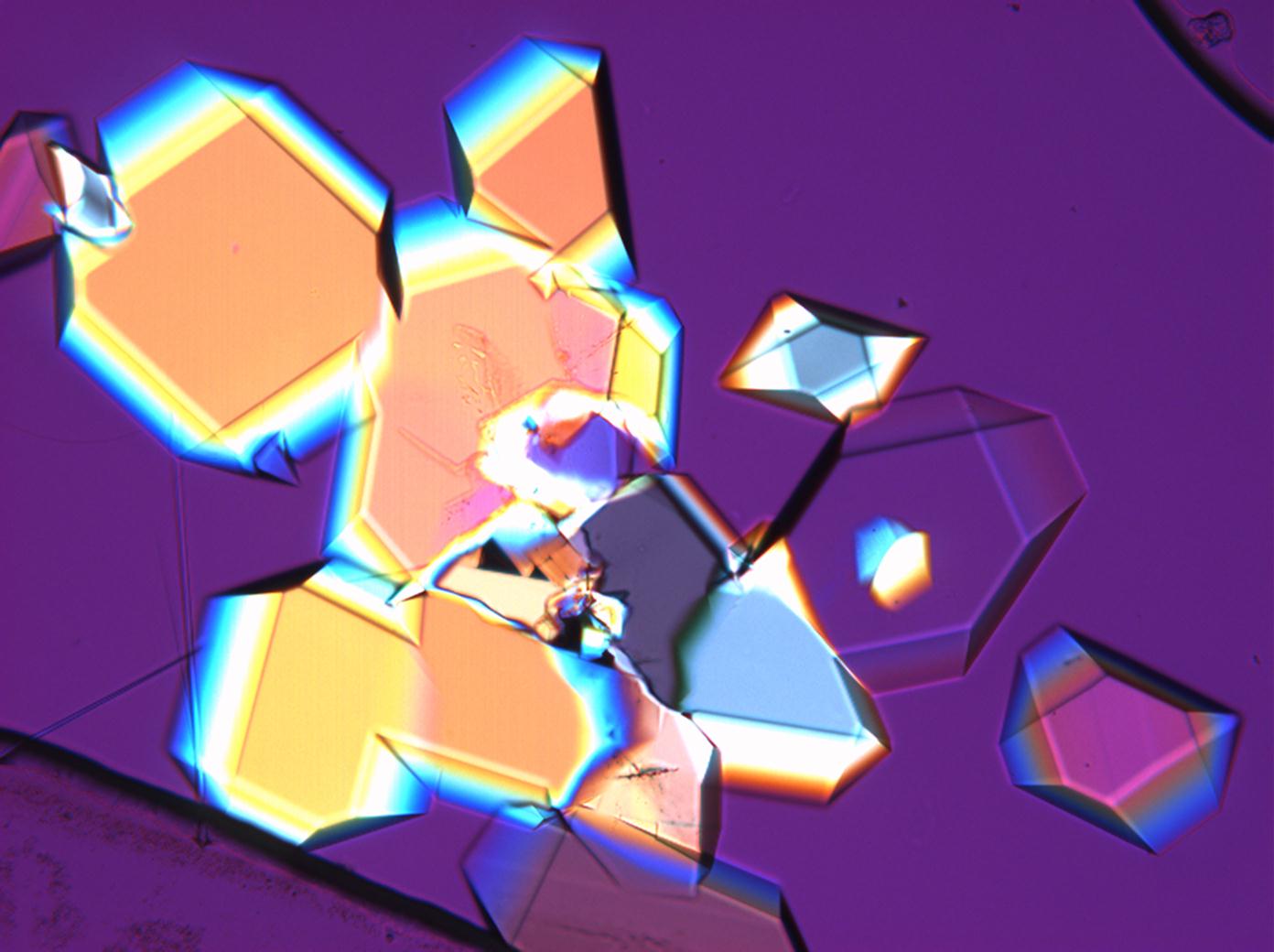
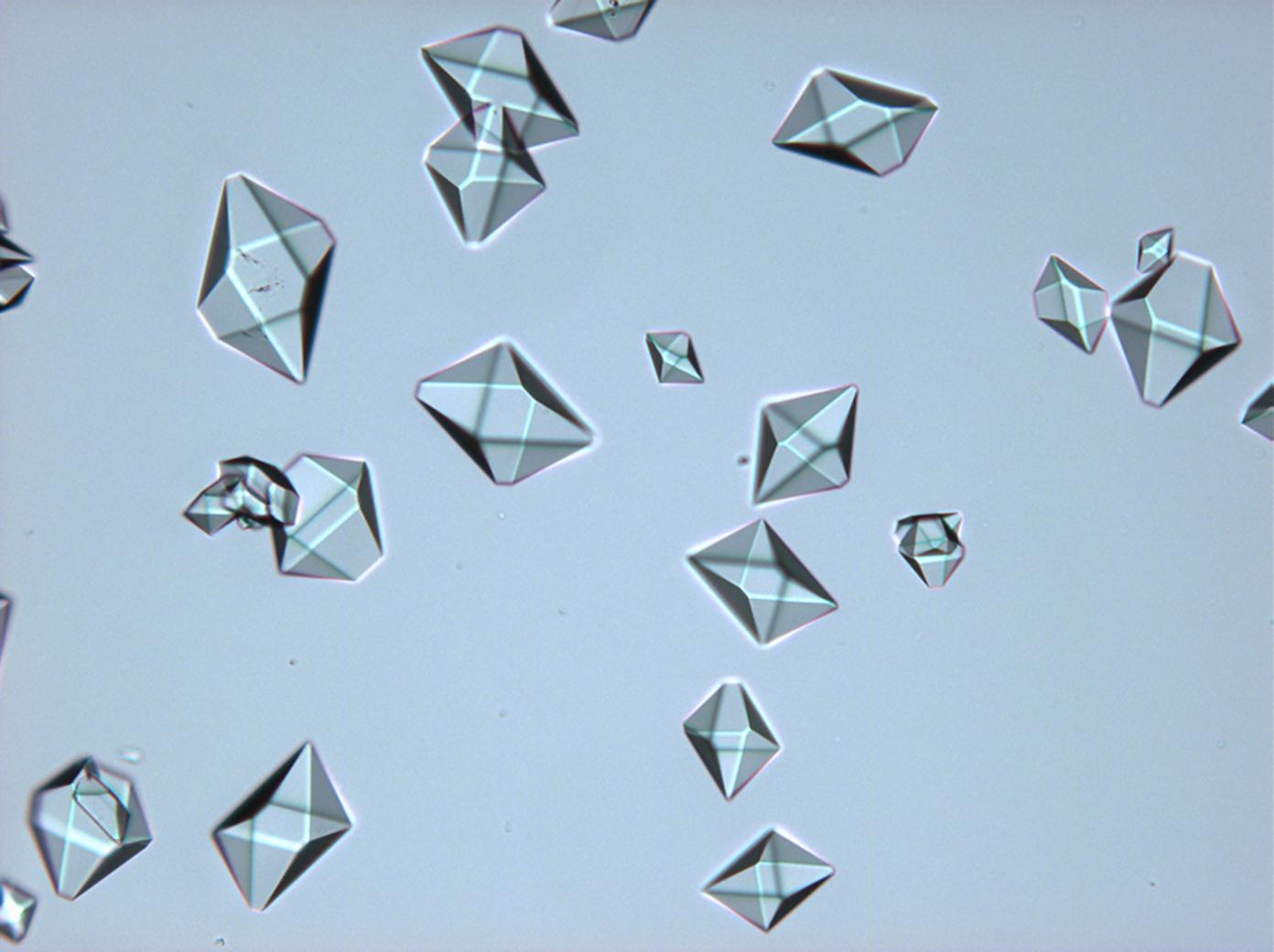
== Pictures of salt and salt damage == | |||
=== Under the polarizing microscope === | |||
<gallery caption="Sodium sulfate crystals between to glass plates " widths="200px" heights="150px" perrow="3"> | |||
Image:HJS Na2SO4-slides-6.jpg |plain polarized light | |||
Image:HJS Na2SO4-slides-1.jpg| crossed polarisers, red I | |||
Image: | | |||
Image:HJS Na2SO4-slides-110703-10x-3.jpg|plain polarized light | |||
Image:HJS Na2SO4-slides-110703-10x-2.jpg| crossed polarisers | |||
Image:HJS Na2SO4-slides-110703-10x-1.jpg| crossed polarisers, red I | |||
Image:HJS Na2SO4-slides-2-110603.jpg|plain polarized light | |||
Image:HJS Na2SO4-slides-1-110603.jpg| crossed polarisers, red I | |||
Image: | | |||
Image:HJS-Na2SO4-111703-02-10x.jpg|plain polarized light | |||
Image:HJS-Na2SO4-111703-04-10x.jpg|plain polarized light | |||
Image:HJS-Na2SO4-111703-01-10x.jpg| crossed polarisers, red I | |||
</gallery> | |||
<br> | |||
<gallery caption="Sodium sulfate crystals, crystallised out of a water extraction of a real sample" widths="200px" heights="150px" perrow="3"> | |||
Image:HJS Na2SO4 092503-3.jpg|plain polarized light | |||
Image:HJS Na2SO4 092503-4.jpg|plain polarized light | |||
</gallery> | |||
<!-- | |||
=== Under the Scanning Electron Microscope === | |||
--> | |||
==Weblinks== | |||
<references/> | |||
==Literature== | |||
<biblist/> | |||
'''Further literature''' | |||
<bibprint filter=" title:%Na2SO4%"/> | |||
[[Category:sodium sulfate]][[Category:Stahlbuhk,Amelie]][[Category:Schwarz,Hans-Jürgen]][[Category:R-MSteiger]][[Category:InProgress]][[Kategorie:Müller,Tim]][[Category:Sulfate]] [[Category:Salt]][[Category:List]] | |||
Revision as of 12:15, 7 May 2015
Authors: Hans-Jürgen Schwarz , Michael Steiger, Tim Müller, Amelie Stahlbuhk
back to Sulfate
| This article will be released soon. |
Abstract[edit]
In this article the different phases of sodium sulfate and their properties will be presented.
Phases and hydrate phases[edit]
There are four different phases of sodium sulfate whereof only two are stable. The others are metastable but were also detected.
Thenardite Na2SO4
Sodium sulfate phase III Na2SO4 metastable
Sodium sulfate heptahydrate Na2SO4•7H2O metastable
Mirabilite Na2SO4•10H2O
Occurrence[edit]
Both thenardite and mirabilite occur as natural minerals. In nature sodium sulfate occurs in mineral waters in the form of double salts, as deposits of former salt lakes. The hydrated sodium sulfate was first described by Glauber in 1658 where he called it "sal mirabilis". Mirabilite is also known as "Glauber's salt" in honor of its discoverer.
Origin and formation of thenardite / mirabilite in monuments[edit]
When sodium ions in conjunction with other anions enter porous inorganic building materials, sodium sulfate may be formed by reaction with sulfate contributed by other sources, for example air contaminated with sulfur oxide gases. Portland cement contains a certain amount of sodium or potassium sulfate. In Germany, the standardization institute (DIN) allows a content of up to 0.5 % soluble alkalis. This means that 100 kg of Portland cement containing only 0.1% soluble Na2O can form 520g of Mirabilite when reacting with sulfate [calculation by Arnold/Zehnder 1991]. Sodium ions can also enter into monuments from various cleaning materials and, in older restoration products, such as water glass. Ground water, and even surface water, are also a possible source of Na+-ions as well as sulfate ions. De-icing road salt may contain a large amount of soluble sodium chloride. Finally, in coastal areas, sea water is a significant source of NaCl.
Solubility[edit]
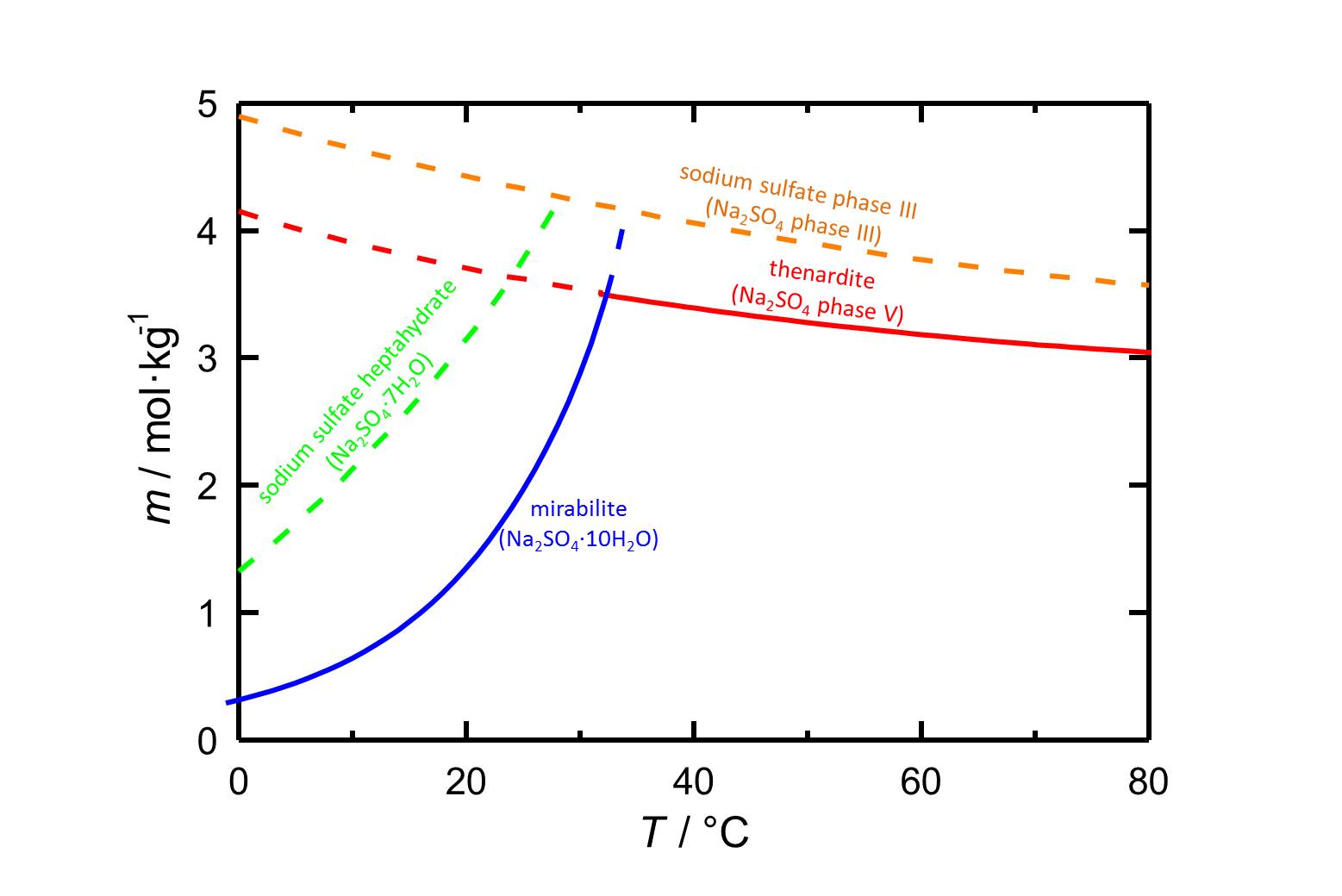
Author: Steiger, Michael; Asmussen, Sönke
 .
.
The phases of sodium sulfate are easily soluble in water (figure 1), so they have a high mobility in porous materials as well. The solubility of the phases depends on the temperature.
The decahydrate mirabilite is stable below 32.4 °C. Above this temperature the anhydrous thenardite is the stable crystalline phase, which in turn is metastable below this transition temperature. In the case of a temperature drop in a solution that is saturated with respect to thenardite, high supersaturations with respect to mirabilite and with that its precipitation are possible, which involves a certain damage potential.
| phase | solubility [mol/kg] at 20°C |
| thenardite | 3.706 |
| sodium sulfate phase III | 4.428 |
| sodium sulfate heptahydrate | 3.143 |
| mirabilite | 1.353 |
Hygroscopicity[edit]
The deliquescence behaviour of the different sodium sulfate phases is shown in figure 2 as a function of temperature. The equilibrium humidities of the thenardite/mirabilite conversion are also represented.
The temperature dependencce for thenardite and for mirabilite are contradictory, as the deliquescence humidity of mirabilite decreases with increasing temperature whereas those of thenardite increases and it is influenced to a lesser extend by temperature changes.
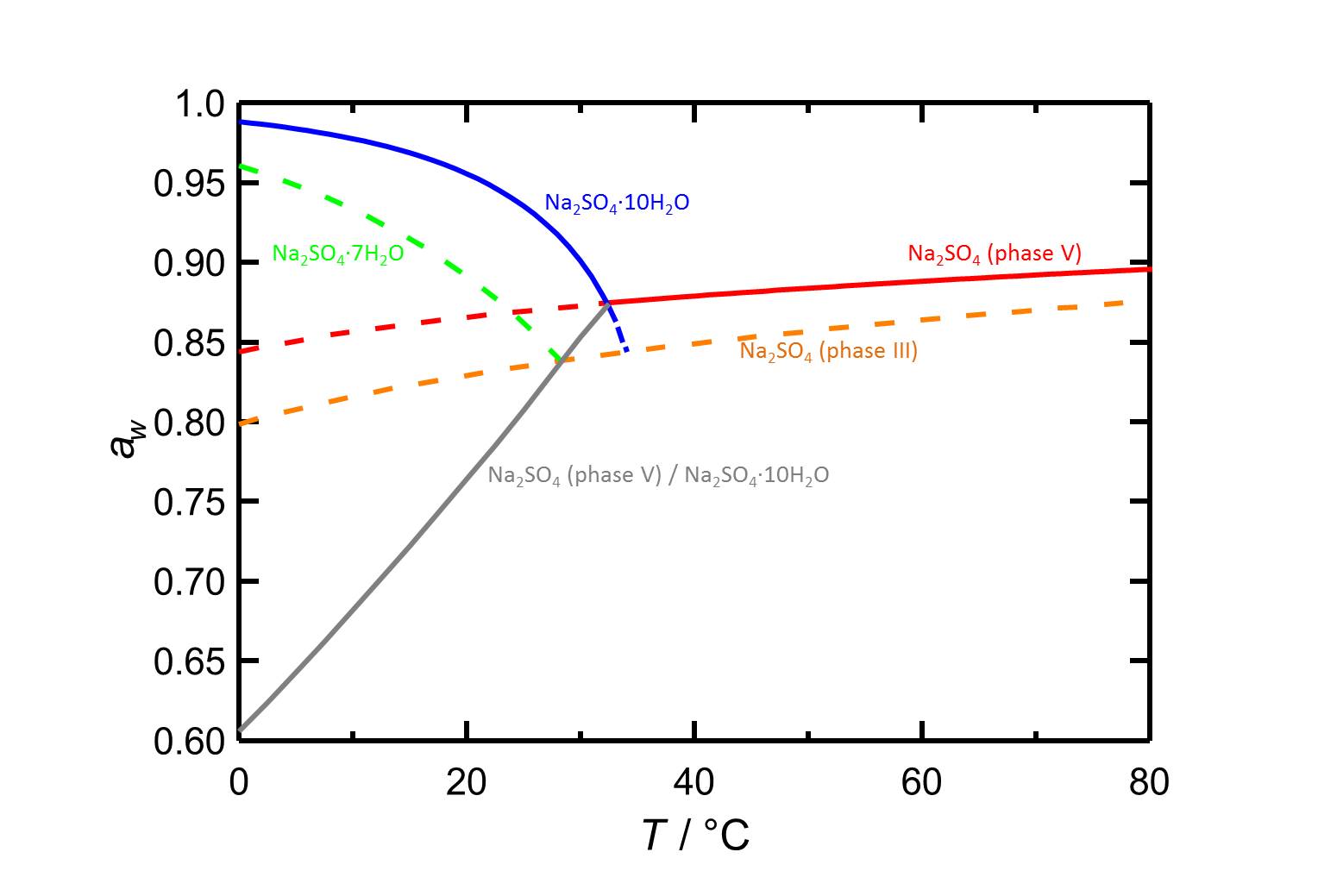
Author: Steiger, Michael; Asmussen, Sönke
 .
.
| considered phase transition | deliquescence or equilibrium humidity at 20 °C |
| sodium sulfate phase III-solution | 82.9 % |
| thenardite-solution | 86.6 % |
| sodium sulfate heptahydrate-solution | 89.1 % |
| mirabilite-solution | 95.6 % |
| thenardite-mirabilite | 76.4 % |
Hydration behavior[edit]
The Na2SO4 – H2O system:
The only stable forms of sodium sulfate are the decahydrate (mirabilite) and the anhydrite (thenardite). The generation of mirabilite by recrystallization of the salt from an aqueous supersaturated solution occurs at 32.4°C. In particular, the transition from thenardite to mirabilite and the incorporation of 10 water molecules in the crystal lattice causes a volume expansion of 320%. This transition happens at a relatively low temperature (32-35°C), the damage caused by this salt is highly dependent on the temperature and thus on the environment. This temperature range is given as a guide, because this transition could happen for example at 25°C at 80% relative humidity, or even at 0°C at 60.7% relative humidity [information from Gmelin]. Due to this strong dependence on the environmental parameters, an estimate of the damage caused on buildings by crystallization and hydration of sodium sulfate are very difficult to obtain.
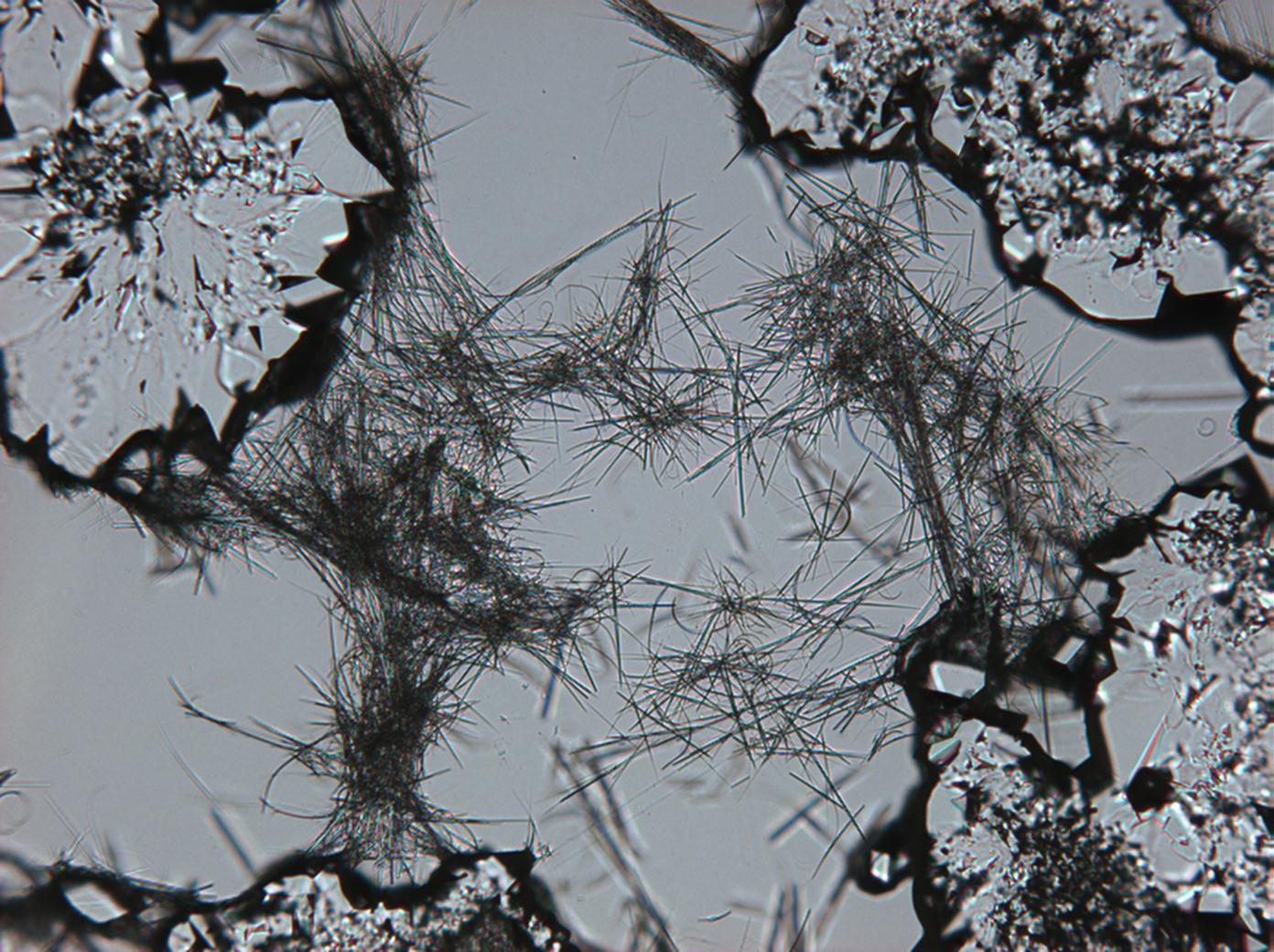
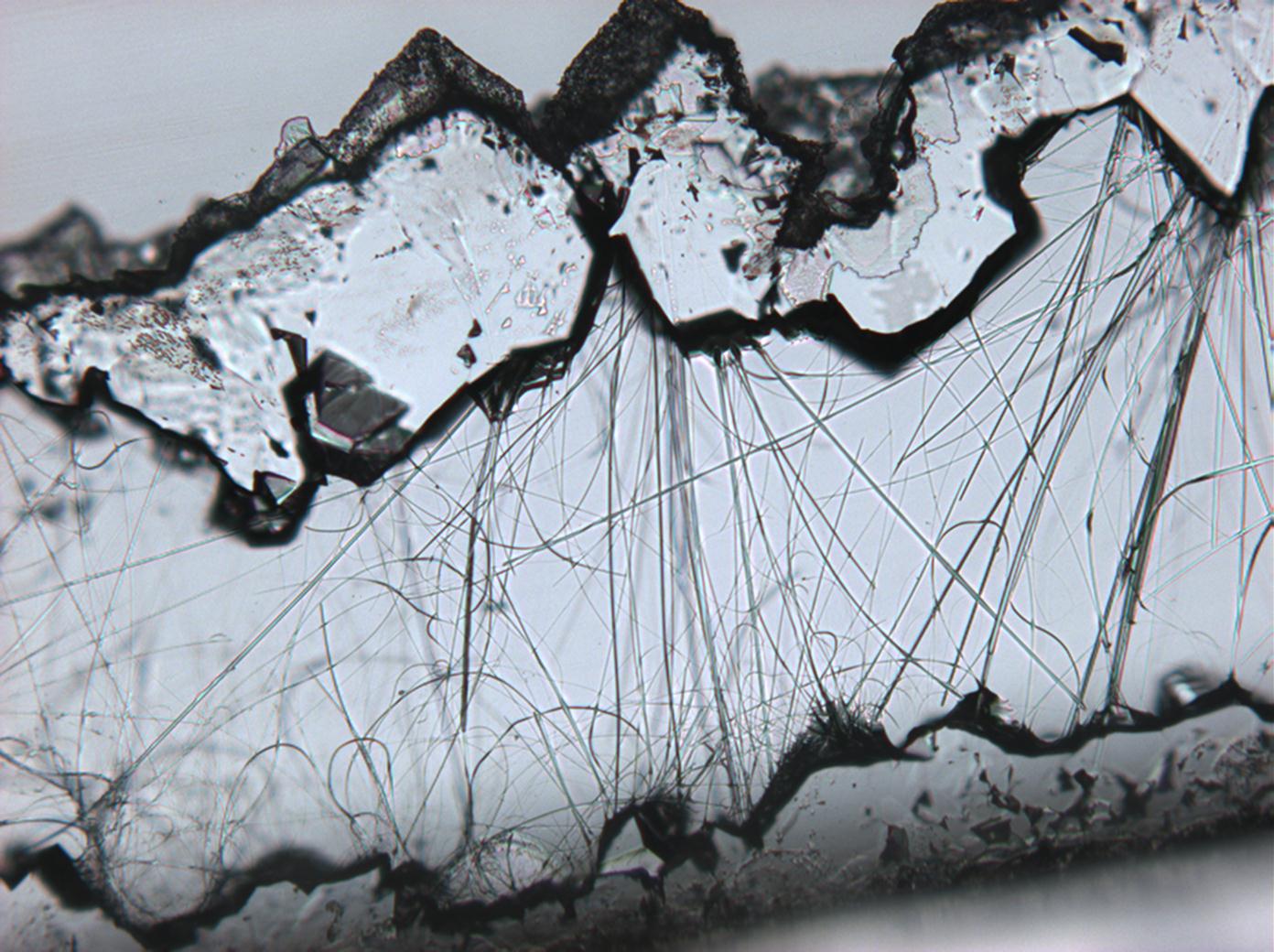
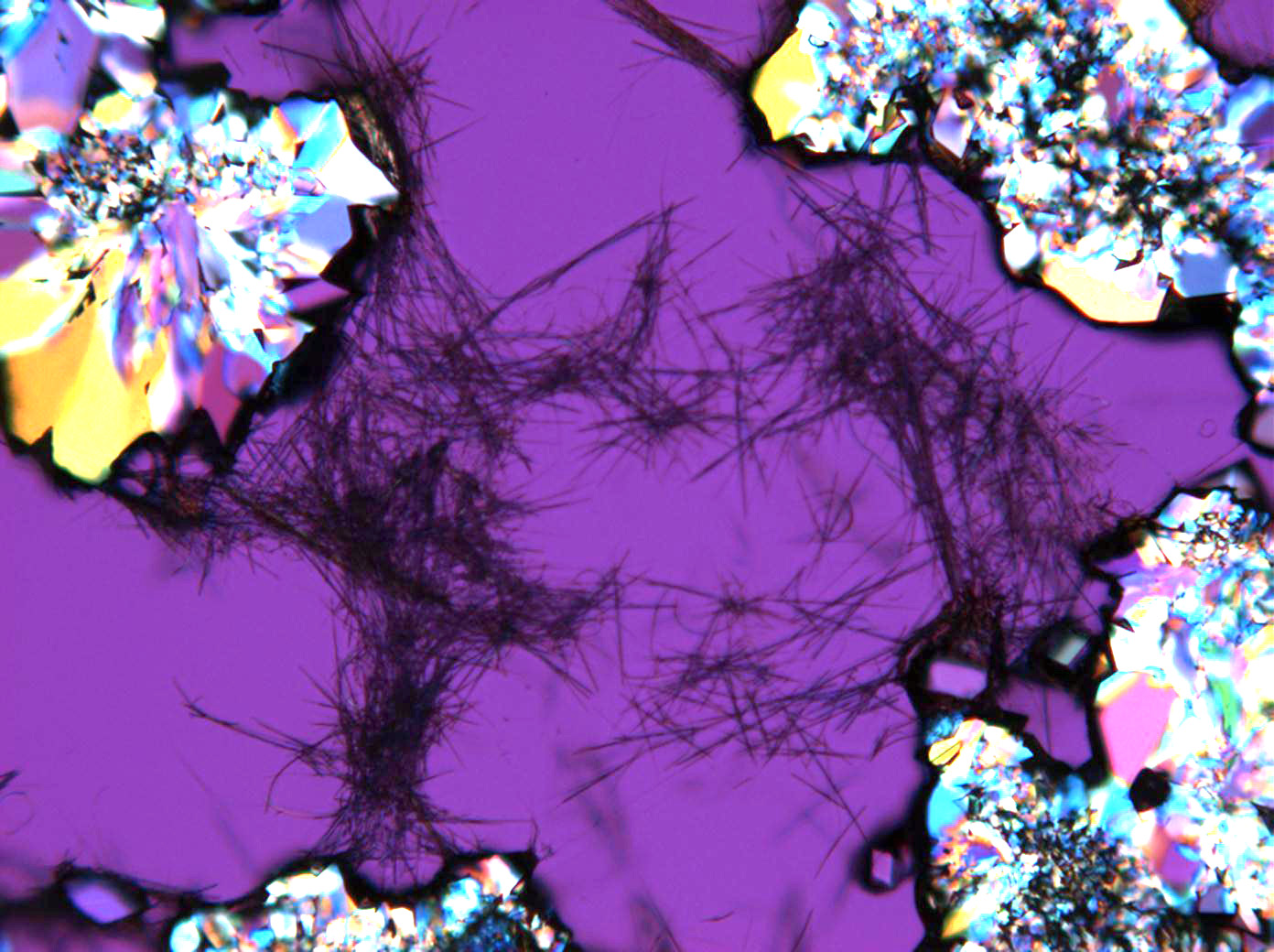
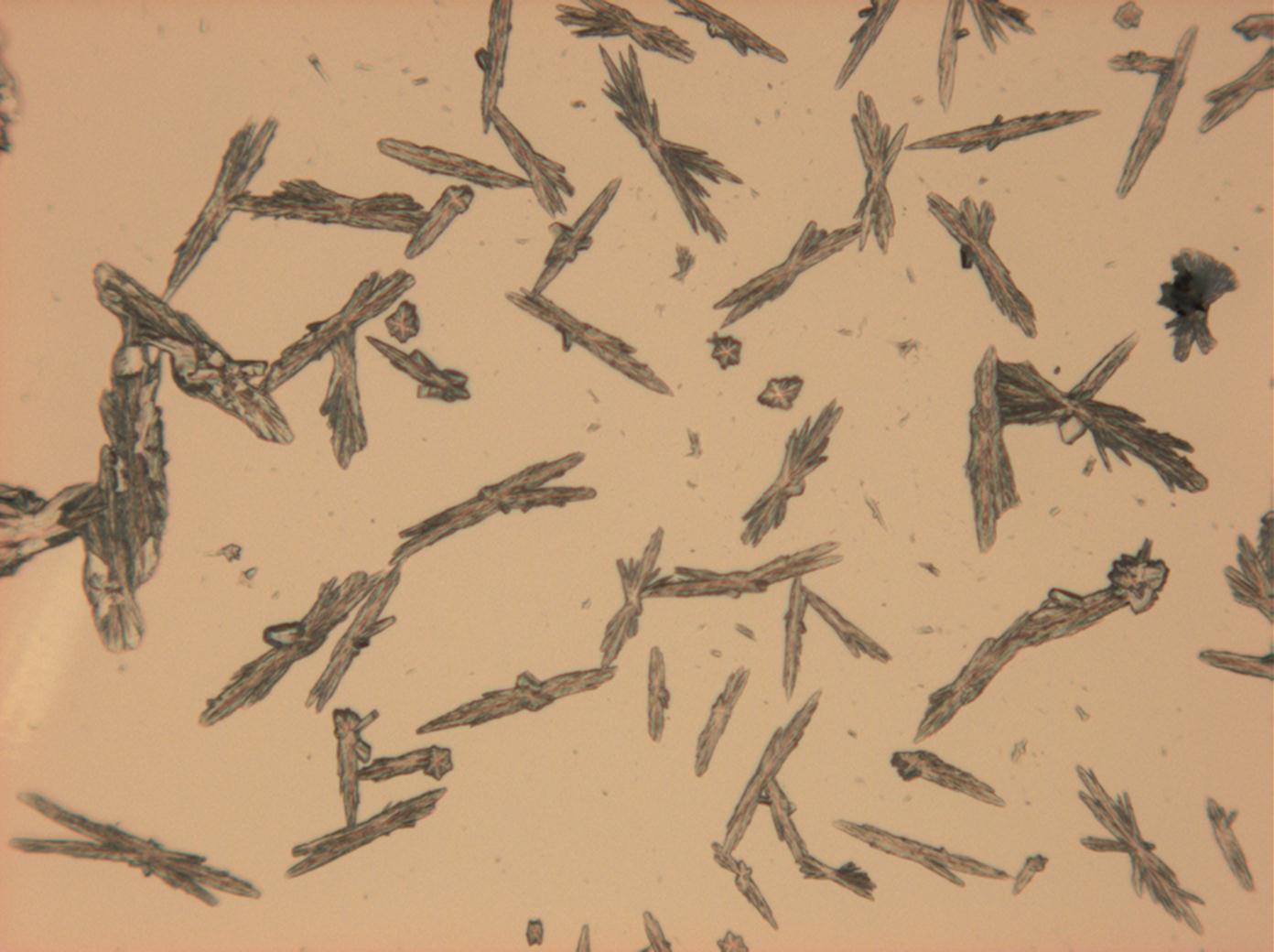
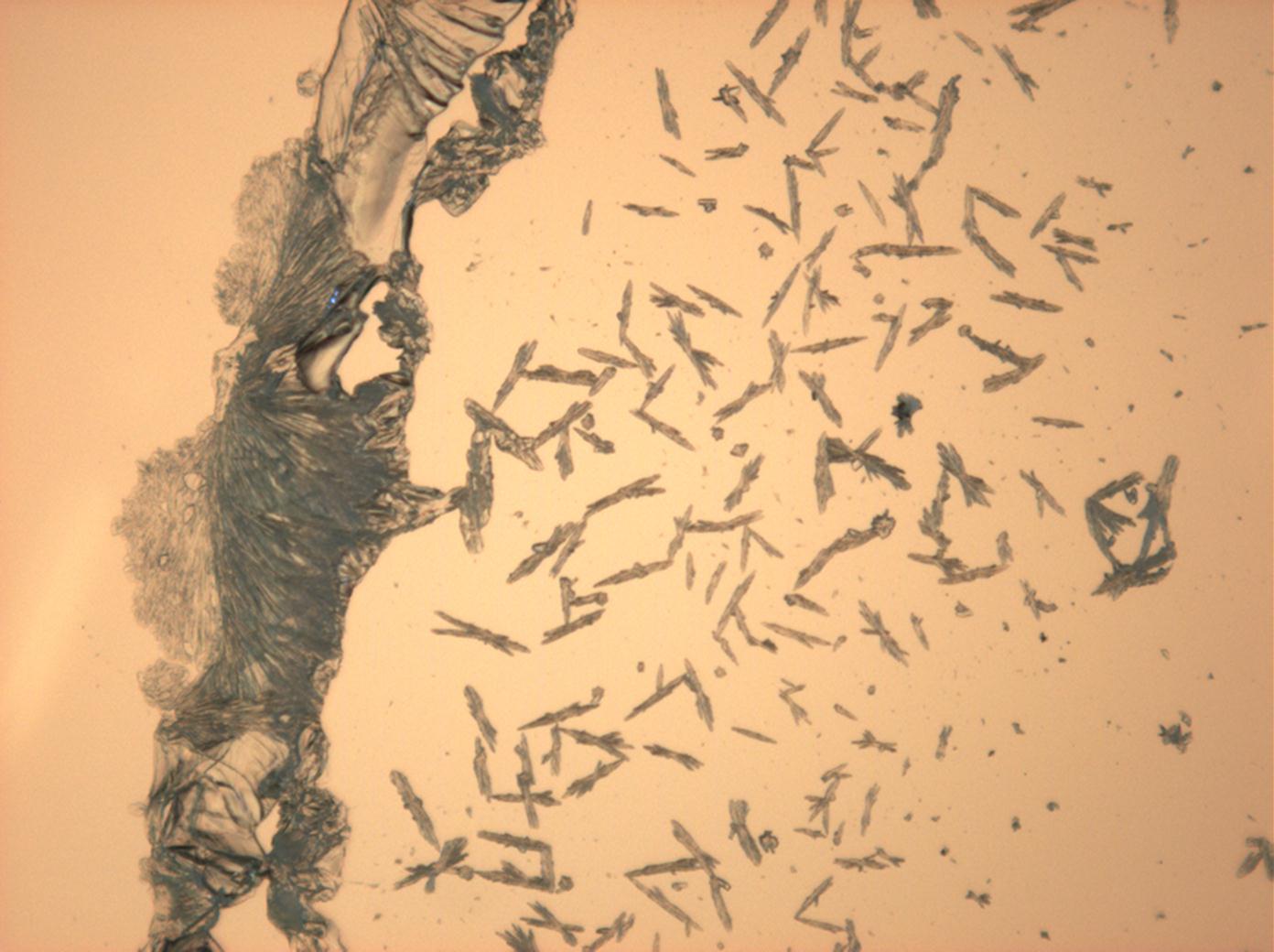
Pictures of salt and salt damage[edit]
Under the polarizing microscope[edit]
- Sodium sulfate crystals between to glass plates
- Sodium sulfate crystals, crystallised out of a water extraction of a real sample
Weblinks[edit]
Literature[edit]
| [Arnold.etal:1991] | Arnold, Andreas; Zehnder, Konrad (1991): Monitoring Wall Paintings Affected by soluble Salts. In: Cather, Sharon (eds.): The Conservation of Wall Paintings: Proceedings of a symposium organized by the Coutrauld Institut of Art and the Getty Conservation Institute, London, July 13-16, The Getty Conservation Institute, 103-136. |  |
| [Saidov:2012] | Saidov, Tamerlan Adamovich (2012): Sodium sulfate heptahydrate in weathering phenomena of porous materials. dissertation, Technische Universiteit Eindhoven, Url |  |
| [Sperling.etal:1980] | Sperling, C.H.B.and Cooke, R.U. (1980): Salt Weathering on Arid Environment, I. Theoretical ConsiderationsII. Laboratory Studies. In: Papers in Geography, 8 () |  |
| [Steiger.etal:2008] | Steiger, Michael; Asmussen, Sönke (2008): Crystallization of sodium sulfate phases in porous materials: The phase diagram Na2SO4–H2O and the generation of stress. In: Geochimica et Cosmochimica Acta, 72 (17), 4291-4306, Url |  |
| [Vogt.etal:1993] | Vogt, R.; Goretzki, Lothar (1993): Der Einfluss hygroskopischer Salze auf die Gleichgewichtsfeuchte und Trocknung anorganischer Baustoffe, unveröffentlichter Bericht. |  |
| [Winkler.etal:1970] | Winkler, Erhard M.; Wilhelm, E.J. (1970): Saltburst by Hydration Pressure in Architectural Stone in Urban Atmosphere. In: Geological Society of America, Bulletin, 81 (), 567-572 |  |
Further literature
| [Amirthalingam.etal:1977] | Amirthalingam V., Karkhanavala M. D., Rao U. R. K. (1977): Topotaxic phase change in Na2SO4. In: Acta Cryst., (), 522 |  |
| [Amirthalingam:1977] | Amirthalingam, V.; Karkhanaavala, M. D.; Rao, U. R. K. (1977): Topotaxic phase change in Na2SO4. In: Acta Cryst., A33 (), 522-523 |  |
| [Bayh.etal:1966] | Bayh, W.; Haussuehl, Siegfried (1966): Elastische und mechanische Eigenschaften von Na2SO4 (Thenardit). In: Acta Crystallogr, 20 (6), 931-932 |  |
| [Brown.etal:2000] | Brown, P. W.; Badger, S. (2000): The distributions of bound sulfates and chlorides in concrete subjected to mixed NaCl, MgSO4, Na2SO4 attack. In: Cem. Concr. Res., 30 (10), 1535-1542 |  |
| [Brown.etal:2001] | Brown, P. W.; Badger, S. (2001): Reply to the discussion by William G. Hime and Stella L. Marusin of the paper "The distribution of bound sulfates and chlorides in concrete to mixed NaCl, MgSO4, Na2SO4 attack". In: Cem. Concr. Res., 31 (7), 1117-1118 |  |
| [DeClercq.etal:2012] | Clercq, Hilde; Jovanović, Maja; Linnow, Kirsten; Steiger, Michael (2012): Performance of limestones laden with mixed salt solutions of Na2SO4–NaNO3 and Na2SO4–K2SO4. In: Environmental Earth Sciences, (), 1-11, Url, 10.1007/s12665-012-2017-0 |  |
| [Freyer.etal:1997] | Freyer, D.; Fischer, St.; Koehnke, K.and Voigt W. (1997): Formation of double salt hydrates: I Hydration of quenched Na2SO4-CaSO4 phases. In: Solid State Ionics, 96 (2), 29-33, 10.1016/S0167-2738(96)00612-1, |  |
| [Friedel:1978] | Friedel, B. (1978): Gipslöslichkeiten in wässerigen Systemen mit NaCl, MgCl2, Na2SO4 und MgSO4. In: Zeitschrift für Pflanzenernährung und Bodenkunde, 141 (3), 337-346, 10.1002/jpln.19781410309 |  |
| [Lindstroem.etal:2014] | Lindström, N.; Talreja, T.; Linnow, K.; Steiger, M. (2014): Crystallization behavior of a Na2SO4–MgSO4 salt mixture and comparison to single salt behavior. In: Hilde De Clercq (eds.): Proceedings of SWBSS 2014 3rd International Conference on Salt Weathering of Buildings and Stone Sculptures,KIK-IRPA, Royal Institute for Cultural Heritage Brussels 151-165, 10.5165/hawk-hhg/258. |   |
| [Lindstroem.etal:2015] | Nadine Lindström; Nicole Heitmann; Kirsten Linnow; Michael Steiger (2015): Crystallization behavior of NaNO3–Na2SO4 salt mixtures in sandstone and comparison to single salt behavior. In: Applied Geochemistry, 63 (), 116 - 132, Url, https://doi.org/10.1016/j.apgeochem.2015.07.007 |  |
| [Lindstroen.etal:2016] | Nadine Lindström; Tanya Talreja; Kirsten Linnow; Amelie Stahlbuhk; Michael Steiger (2016): Crystallization behavior of Na2SO4–MgSO4 salt mixtures in sandstone and comparison to single salt behavior. In: Applied Geochemistry, 69 (), 50 - 70, Url, https://doi.org/10.1016/j.apgeochem.2016.04.005 |  |
| [Linnow.etal:2012] | Linnow, Kirsten; Steiger, Michael; Lemster, Christine; Clercq, Hilde; Jovanović, Maja (2012): In situ Raman observation of the crystallization in NaNO3–Na2SO4–H2O solution droplets. In: Environmental Earth Sciences, (), 1-12, Url, 10.1007/s12665-012-1997-0 |  |
| [Marliacy.etal:2000] | Marliacy, P.; Solimando, R.; Bouroukba, M.; Schuffenecker, L. (2000): Thermodynamics of crystallization of sodium sulfate decahydrate in H2O-NaCl-Na2SO4: application to Na2SO4.cntdot.10H2O-based latent heat storage materials. In: Thermochim. Acta, 344 (1), 85-94 |  |
| [Moffadel.etal:1991] | Moffadel, N.; Bouzaziz, R.; Mayer, M. (1991): Le polymorphisme du sulfate de sodum anhydre et les phases intermédiaries, glasérite et aphtitalite, dans le binaire Na2SO4-K2SO4. In: Thermochimica Acta, 185 (1), 141-153, 10.1016/0040-6031(91)80125-3 |  |
| [Naruse.etal:1987] | Naruse, H.; Tanaka, K.; Morikawa, H.; Marumo, F. (1987): Structure of Na2SO4(I) at 693 K. In: Acta Crystallographica, B43 (), 143-146 |  |
| [Nord:1973] | Nord, Anders G. (1973): Refinement of the Crystal Structure of Thenardite, Na2SO4(V). In: Acta Chem. Scand., 27 (3), 814-822 |  |
| [Platford:1975] | Platford, R. F. (1975): Thermodynamics of the system H2O-NaCl-MgCl2-Na2SO4-MgSO4 at 25 degrees C. In: Mar. Chem., 3 (4), 261-270 |  |
| [Potter.etal:1978] | Potter, R. W. I.; Clynne, M. A. (1978): Solubility of high soluble salts in aqueous media; Part 1, NaCl, KCl, CaCl2, Na2SO4, and K2SO4 solubilities to 100 degrees C. In: Journal of Research of the U. S. Geological Survey, 6 (6), 701-705 |  |
| [Ptacek.etal:1992] | Ptacek, C. J.; Reardon, E. J. (1992): Solubility of siderite (FeCO3) in concentrated NaCl and Na2SO4 solutions at 25 degrees C. In: Kharaka, Yousif K.; Maest, Ann S. (eds.): Proceedings of the 7th international symposium on water-rock interaction, 181-184. |  |
| [Rard:1979] | Rard, J. A.; Miller, D. G. (1979): The mutual diffusion coefficients of Na2SO4-H2O and MgSO4-H2O at 25 degrees C from Rayleigh interferometry. In: J. Solut. Chem., 8 (10), 755-756 |  |
| [Rasmussen.etal:1996] | Rasmussen, Svend Erik; Jorgensen, Jens-Erik; Lundoft, Britta (1996): Structure and phase transition of Na2SO4. In: Journal of Applied Crystallography, 29 (), 42-47, 10.1107/S0021889895008818 |  |
| [Sarada.etal:1990] | Sarada, S.; Ananthaswamy, J. (1990): Thermodynamic Properties of Electrolyte Solutions: Emf Study of the System NaCl-Na2SO4-H20 at 25, 35 and 45 ÄC. In: Journal Chem. Soc. Faraday Trans., 86 (1), 81-84 |  |
| [Steiger.etal:2008] | Steiger, Michael; Asmussen, Sönke (2008): Crystallization of sodium sulfate phases in porous materials: The phase diagram Na2SO4–H2O and the generation of stress. In: Geochimica et Cosmochimica Acta, 72 (17), 4291-4306, Url |  |
| [Tishchenko.etal:1992] | Tishchenko, Pavel Ya.; Bychkov, Alexander S.; Hravéczy-Páll, Andrea; Tóth, Klára; Pungor, Ernoe (1992): Activity Coefficients for the System NaCl + Na2SO4 + H2O at Various Temperatures. Application of Pitzer's Equations. In: Journal of Solution Chemistry, 21 (3), 261-274 |  |
| [Xu.etal:1999] | Xu B., Schweiger G. (1999): In-situ Raman observations of phase transformation of Na2SO4 during the hydration/dehydration cycles on single levitated microparticle.. In: J. Aerosol. Sci., (), 379-380 |  |
| [Zdanovskii.etal:1991] | Zdanovskii, A. B.; Frolovskii, E. E. (1991): Equations for calculating the solubility of mirabilite in the aqueoussodium chloride-magnesium sulfate (2NaCl + MgSO4 = Na2SO4 + MgCl2) system at 0-25.degree. In: Zh. Prikl. Khim. (Leningrad), 64 (6), 1153-7 |  |