Calcium chloride
Author: Amelie Stahlbuhk
back to Chloride
| This article will be released soon. |
Abstract[edit]
The different hydrate stages of calcium chloride are presented, as well as their behavior regarding solubility and hygroscopicity.
Hydrate stages[edit]
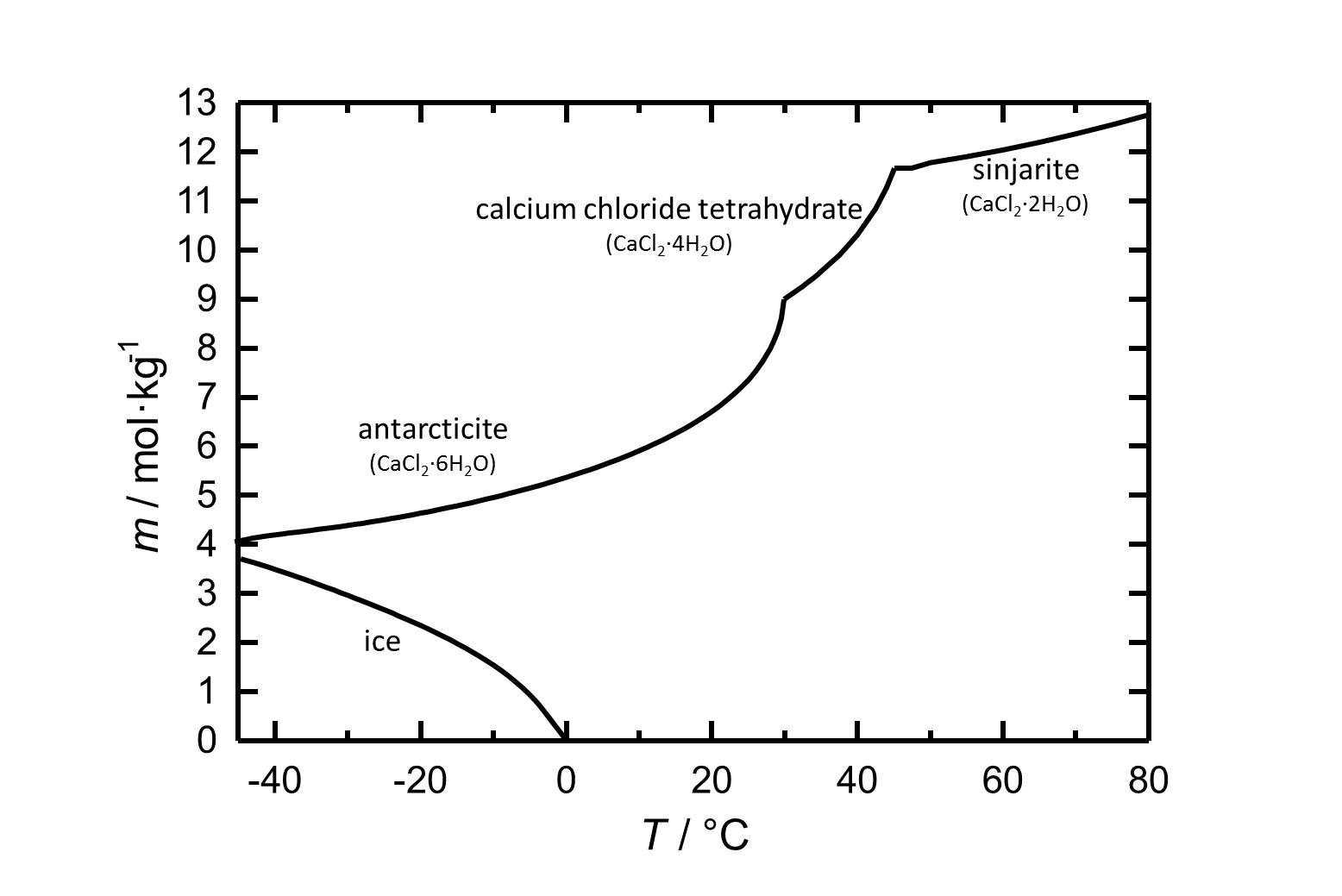
Sinjarite: CaCl2•2H2O
Calcium chloride tetrahydrate: CaCl2•4H2O
Antarcticite: CaCl2•6H2O
Solubility[edit]
Under standard conditions the hexahydrate of calcium chloride Antarcticite is the stable form. The salt has got a high solubility in water which increases with increasing temperatures. The dehydration steps to the calcium chloride tetrahydrate and to sinjarite take place at temperatures of 30 °C and 45 °C, respectively.
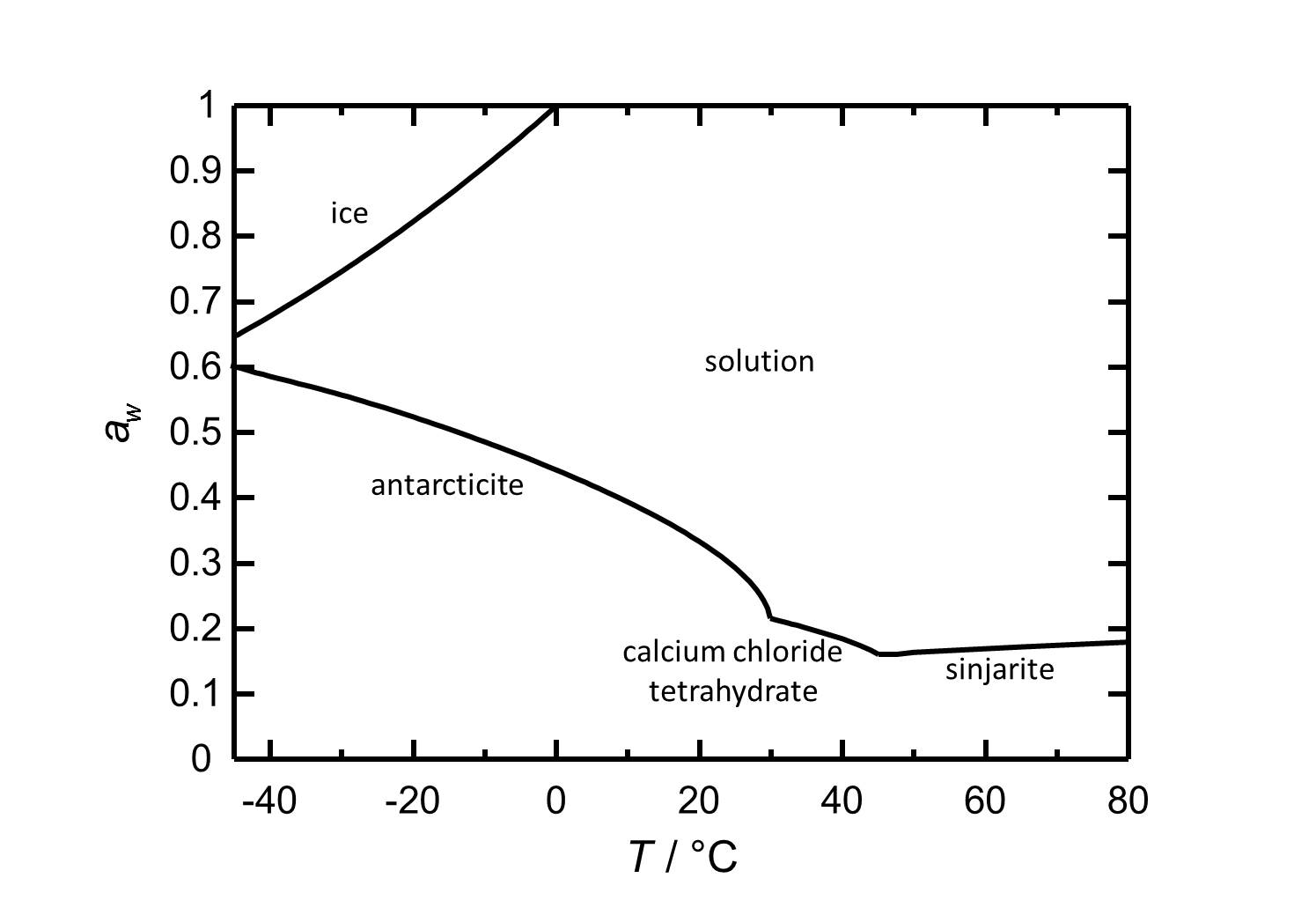
Hygroscopicity[edit]
At room temperature the present Antarcticite has got a deliquescence humidity of about 30 %. At temperatures up to 30 °C Antarcticite is also stable at very low relative humidities, a fact that shows the hygroscopicity of the phase. At room temperature changes in the relative humidity do not provoke phase transitions. Calcium chloride tetrahydrate and Sinjarite constantly have deliquescence humidities below 22 % in their temperature ranges.