Calcium chloride
Author: Amelie Stahlbuhk
back to Chloride
Abstract[edit]
The different hydrates of calcium chloride are presented, as well as their behavior regarding solubility and hygroscopicity.
Hydrate stages[edit]
Calcium chloride monohydrate: CaCl2•H2O
Sinjarite: CaCl2•2H2O
Calcium chloride tetrahydrate: CaCl2•4H2O
Antarcticite: CaCl2•6H2O
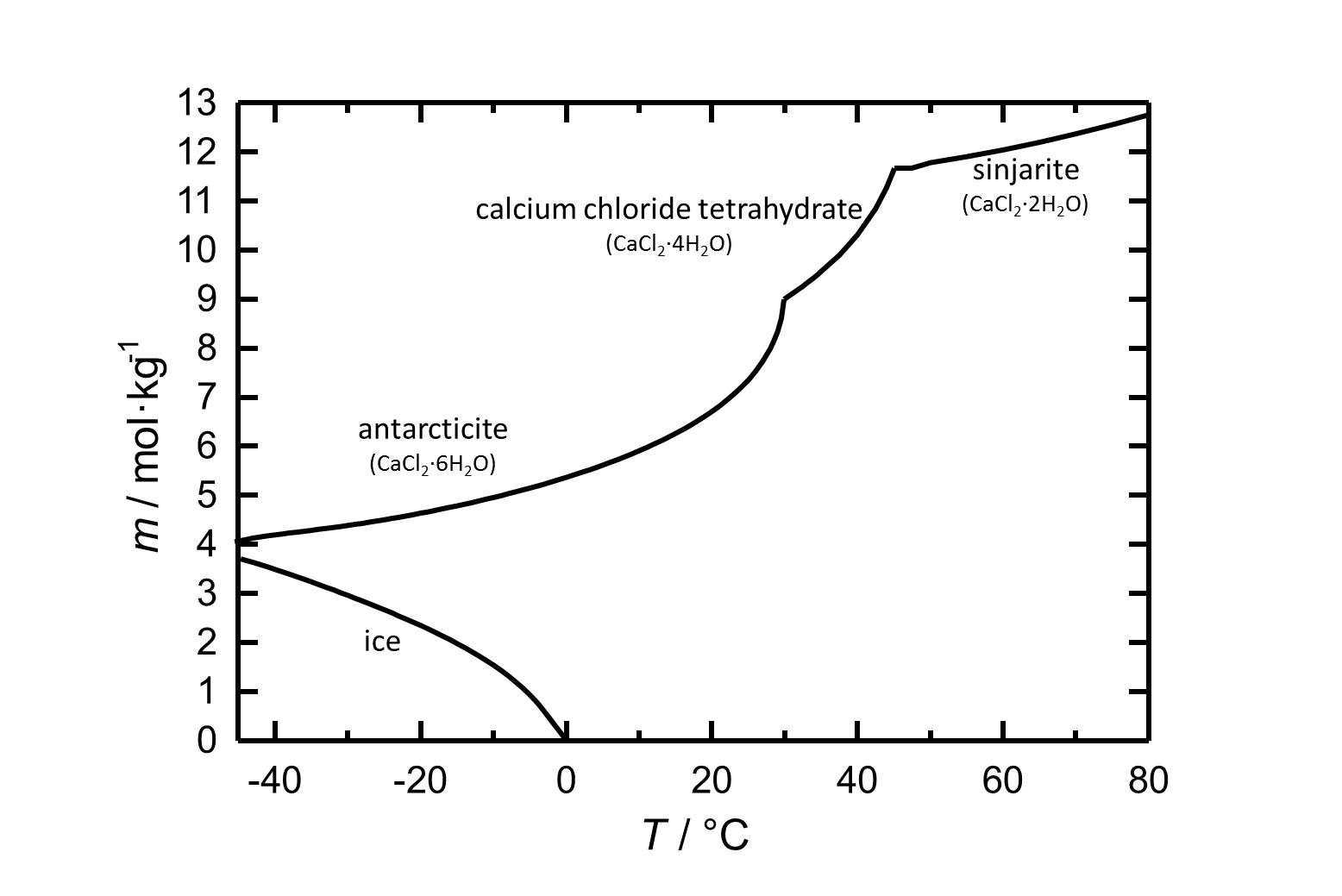
Solubility[edit]
Under standard conditions the hexahydrate of calcium chloride Antarcticite is the stable form. The salt has got a high solubility in water which increases with increasing temperatures. The dehydration steps to the calcium chloride tetrahydrate and to sinjarite take place at temperatures of 30 °C and 45 °C, respectively.
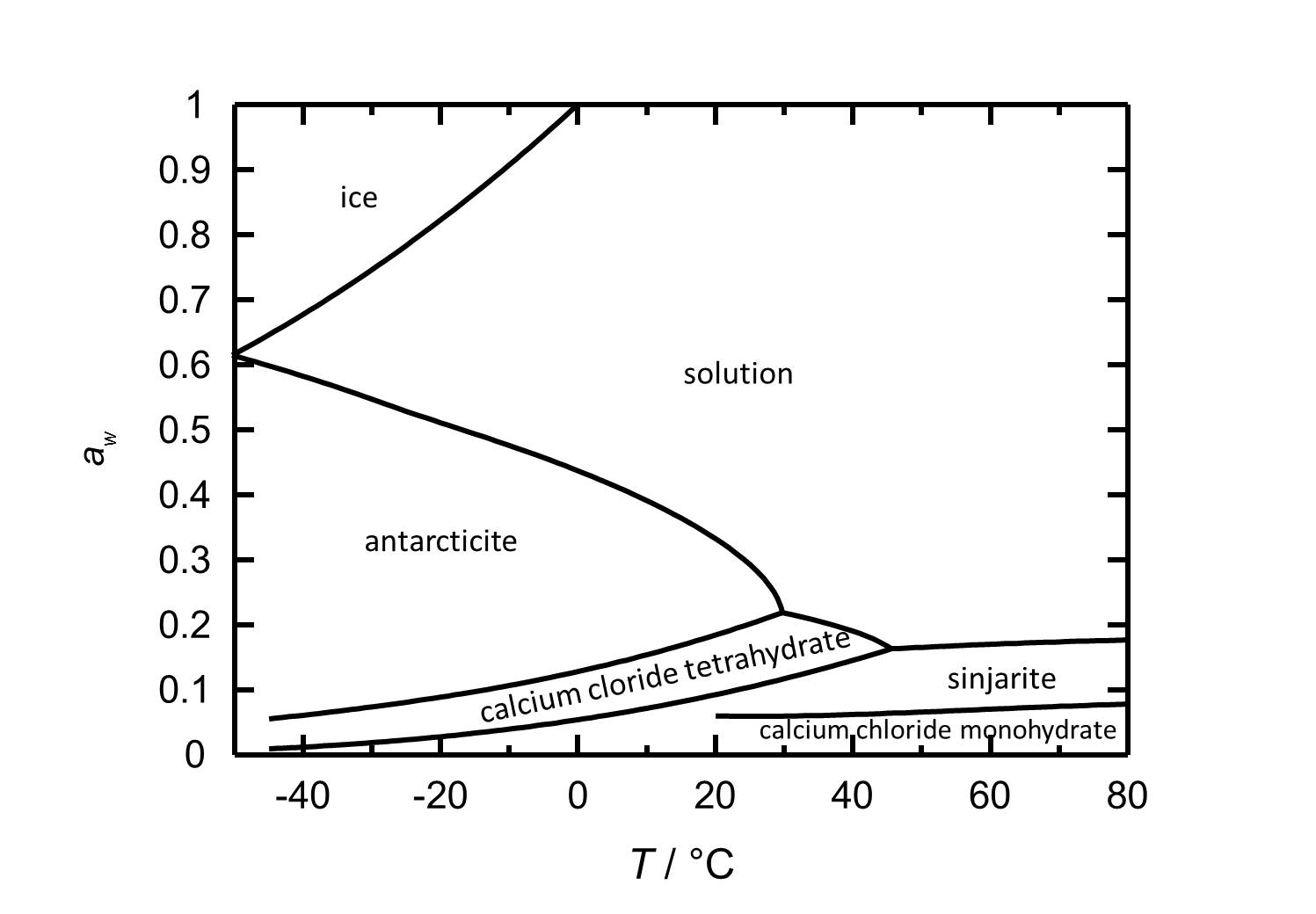
Hygroscopicity[edit]
The hydration/dehydration and crystallization/deliquescence processes in the CaCl2-H2O system can occur by either changing relative humidity or temperature. The deliquescence relative humidity decreases with increasing temperature. At room temperature (20ºC), the hexahydrate [Antarcticite]] is the stable phase, having a deliquescence humidity of about 30% RH. Lowering the relative humidity at the same temperature, calcium chloride tetrahydrate forms at values below 18% RH, while dehydration to the dihydrate (sinjarite) occurs at 9% RH, while the latter changes to the monohydrate at 6% RH.
| Phase transition | Deliquescence or equilibrium humidity at 20°C |
| Antarcticite-solution | 33.3 % |
| Antarcticite-Calcium chloride tetrahydrate | 18.5 % |
| Calcium chloride tetrahydrate-Sinjarite | 9 % |
| Sinjarite-Calcium chloride monohydrate | 6 % |