Nitromagnesite: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
No edit summary |
||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{Infobox_Salt | {{Infobox_Salt | ||
|Footnote = <ref>http://www.mindat.org/min-2920.html seen on 29.07.2010 | |Footnote = <ref>http://www.mindat.org/min-2920.html seen on 29.07.2010</ref> | ||
|photo = [[File:Mg-NO3-2 (1).jpg|300px]] | |photo = [[File:Mg-NO3-2 (1).jpg|300px]] | ||
|mineralogical_Name = Nitromagnesite | |mineralogical_Name = Nitromagnesite | ||
| Line 10: | Line 10: | ||
|Crystal_Structure = | |Crystal_Structure = | ||
|Deliqueszenzhumidity = 55.7% | |Deliqueszenzhumidity = 55.7% | ||
|Solubility = | |Solubility = 4.73 mol/kg | ||
|Density = 1.63 g/cm<sup>3</sup> | |Density = 1.63 g/cm<sup>3</sup> | ||
|MolVolume = 157.7 cm<sup>3</sup>/mol | |MolVolume = 157.7 cm<sup>3</sup>/mol | ||
| Line 26: | Line 26: | ||
|chemBehavior = soluble in alcohol | |chemBehavior = soluble in alcohol | ||
|Comments = | |Comments = | ||
|Literature = <bib id="Steiger.etal:2014"/><bib id="Broul.etal:1981"/> | |||
}} | }} | ||
back to [[Nitrate]] | back to [[Nitrate]] | ||
==Solubility== | |||
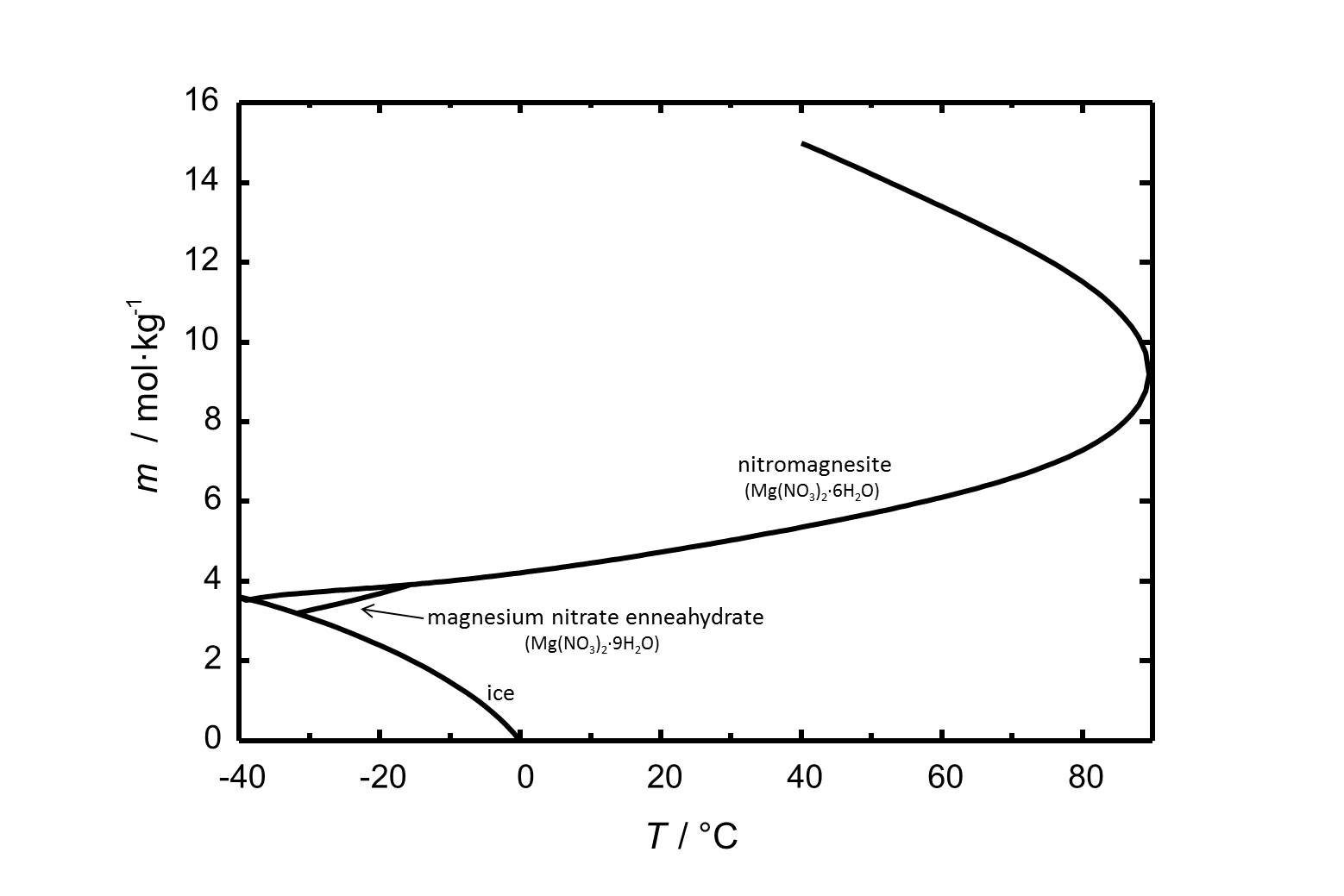
Nitromagnesite has got a solubility of 4.7 mol/kg (at 20 °C). The solubility of nitromagnesite and other phases of the system Mg(NO<sub>3</sub>)<sub>2</sub>-H<sub>2</sub>O is shown in Fig. 1. | |||
<br> | |||
[[Image:Mg(NO3)2 S.jpg|thumb|left|800px|Figure 1: Solubility of magnesium nitrate in water. The molality ''m'' [n(Mg(NO<sub>3</sub>)<sub>2</sub>)•kg(H<sub>2</sub>O)<sup>-1</sup>] is plotted versus the temperature.]] | |||
<br clear=all> | |||
==Hygroscopicity== | ==Hygroscopicity== | ||
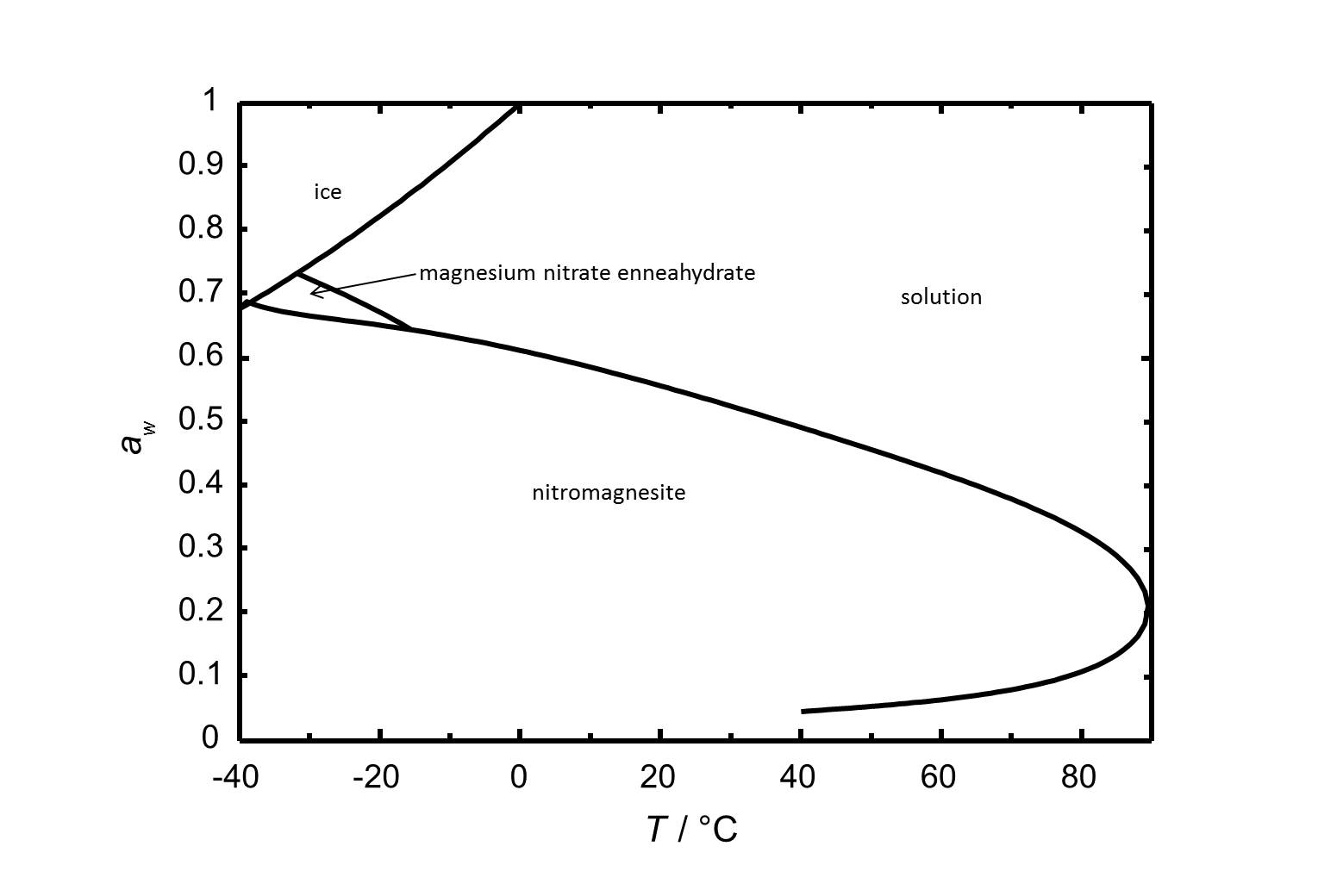
The phase diagram of the system Mg(NO<sub>3</sub>)<sub>2</sub>-H<sub>2</sub>O is shown in Fig. 2.<br> | |||
[[Image:D Mg(NO3)2 e.jpg|thumb|left|800px|Figure 1: Phase diagram of the system Mg(NO<sub>3</sub>)<sub>2</sub>-H<sub>2</sub>O. The water activity ''a<sub>w</sub>'' is plotted versus is plotted versus the temperature.]] | |||
<br clear=all> | |||
<br clear=all> | <br clear=all> | ||
| Line 60: | Line 74: | ||
<biblist/> | <biblist/> | ||
[[Category:Nitromagnesite]][[Category:Nitrate]][[Category:Salt]][[Category:InProgress]] | [[Category:Nitromagnesite]][[Category:Nitrate]][[Category:Salt]][[Category:InProgress]][[Category:List]] | ||
Latest revision as of 11:50, 3 May 2023
| Nitromagnesite[1] | |

| |
| Mineralogical name | Nitromagnesite |
| Chemical name | Magnesium Nitrate Hexahydrate |
| Trivial name | |
| Chemical formula | Mg(NO3)2•6H2O |
| Other forms | |
| Crystal system | monoclinic |
| Crystal structure | |
| Deliquescence humidity 20°C | 55.7% |
| Solubility (g/l) at 20°C | 4.73 mol/kg |
| Density (g/cm³) | 1.63 g/cm3 |
| Molar volume | 157.7 cm3/mol |
| Molar weight | 256.41 g/mol |
| Transparency | transparent |
| Cleavage | perfect |
| Crystal habit | |
| Twinning | |
| Phase transition | |
| Chemical behavior | soluble in alcohol |
| Comments | |
| Crystal Optics | |
| Refractive Indices | nx = 1.34 ny = 1.506 nz = 1.506 |
| Birefringence | Δ = 0.166 |
| Optical Orientation | negative |
| Pleochroism | |
| Dispersion | |
| Used Literature | |
| [Steiger.etal:2014]Title: Weathering and Deterioration Author: Steiger, Michael; Charola A. Elena; Sterflinger, Katja  [Broul.etal:1981]Title: Solubility in organic two component systems [Broul.etal:1981]Title: Solubility in organic two component systemsAuthor: Broul M., Nyvlt J.; Soehnel O. 
| |
back to Nitrate
Solubility[edit]
Nitromagnesite has got a solubility of 4.7 mol/kg (at 20 °C). The solubility of nitromagnesite and other phases of the system Mg(NO3)2-H2O is shown in Fig. 1.
Hygroscopicity[edit]
The phase diagram of the system Mg(NO3)2-H2O is shown in Fig. 2.
| 0°C | 10°C | 20°C | 30°C | 40°C | 50°C |
| 61.3%r.h. | 58.6%r.h. | 55.7%r.h. | 52.5%r.h. | 49.2%r.h. | 45.7%r.h. |
References[edit]
- ↑ http://www.mindat.org/min-2920.html seen on 29.07.2010
Literature[edit]
| [Broul.etal:1981] | Elsevier (eds.) Broul M., Nyvlt J.; Soehnel O. (1981): Solubility in organic two component systems, Elsevier |  |
| [Steiger.etal:2014] | Steiger, Michael; Charola A. Elena; Sterflinger, Katja (2014): Weathering and Deterioration. In: Siegesmund S.; Snethlage R. (eds.): Stone in Architecture, Springer Verlag Berlin Heidelberg, 223-316, 10.1007/978-3-642-45155-3_4. |  |