Physical Principles of Moisture: Difference between revisions
No edit summary |
No edit summary |
||
| Line 78: | Line 78: | ||
Water absorption can be measured with test tubes according to [[Test Tube KARSTEN | KARSTEN]], MIROWSKI or PLEYERS. | Water absorption can be measured with test tubes according to [[Test Tube KARSTEN | KARSTEN]], MIROWSKI or [[Test Tube |PLEYERS]]. | ||
Revision as of 10:19, 23 July 2023
Author: Hans-Jürgen Schwarz
back to Moisture
Abstract[edit]
The material moisture content indicates the amount of free water contained in a solid material.[1] This has an impact on salt and moisture damage to cultural objects, and should always be considered or examined/determined during condition investigations. In order to gain a better overview and understanding of material moisture, the physical principles of material moisture are presented below.
Equilibrium Moisture[edit]
Similar to the equilibrium moisture content of a salt, the equilibrium moisture content of a material sample can be defined as follows:
| The equilibrium moisture content is the mass-related material moisture content of a material, related to constant boundary conditions, which adjusts with time. |
The sample then becomes neither wetter nor drier. It is therefore clear that determining the equilibrium moisture content can be more important than determining the actual water content. With many materials, a change in humidity causes stretching or shrinking and thus leads to damage. If the humidity of the ambient air deviates from the equilibrium humidity, the new equilibrium humidity is re-established through moisture absorption or moisture release. The equilibrium moisture content of a sample decreases with increasing temperature.
Vapor sorption[edit]
If a dried porous object is stored in an outdoor climate, it will initially increase in mass and after reaching a first final value, this mass will change depending on the relative humidity. With increasing air humidity, the mass of the object also increases; with decreasing air humidity, its mass also decreases. The reason for this is the absorption or release of moisture from or to the surrounding air. Water molecules are deposited on the inside of the surfaces. Especially in very small capillaries, with micropores with a diameter of less than 0.1µm, this process can lead to complete water saturation (capillary condensation). In addition to the material properties, the salt content of a sample particularly plays a major role in water vapour sorption.
| The term vapour sorption refers to the uptake of water from the gaseous phase. |
To characterise the moisture behaviour of building materials, samples are stored at constant temperature (i.e. isothermal conditions) at different humidities. These tests provide the so-called moisture sorption isotherms. The moisture sorption isotherm is the graphical representation of the sorption behaviour of a material. It represents the relationship between the water content of the material and the relative humidity of the surrounding air (equilibrium) at a certain temperature.
Hygroscopic moisture absorption[edit]
In many cases, the moisture content of a building material is significantly determined by the content of hygroscopic salts. The hygroscopic moisture absorption is the water absorption of the building material from the surrounding air. The higher the relative humidity and the higher the salt content of the building material, the more moisture is absorbed. A salt-containing wall can absorb many times more water from the surrounding air than the so-called equilibrium moisture or sorption moisture of the salt-free building material.
Hygroscopic moisture absorption refers to the absorption of water from the gas phase of the air by a hygroscopic salt.
Sorption isotherm[edit]
| The sorption isotherm is the graphical representation of the sorption behavior of a material as a function of relative humidity at constant temperature. |
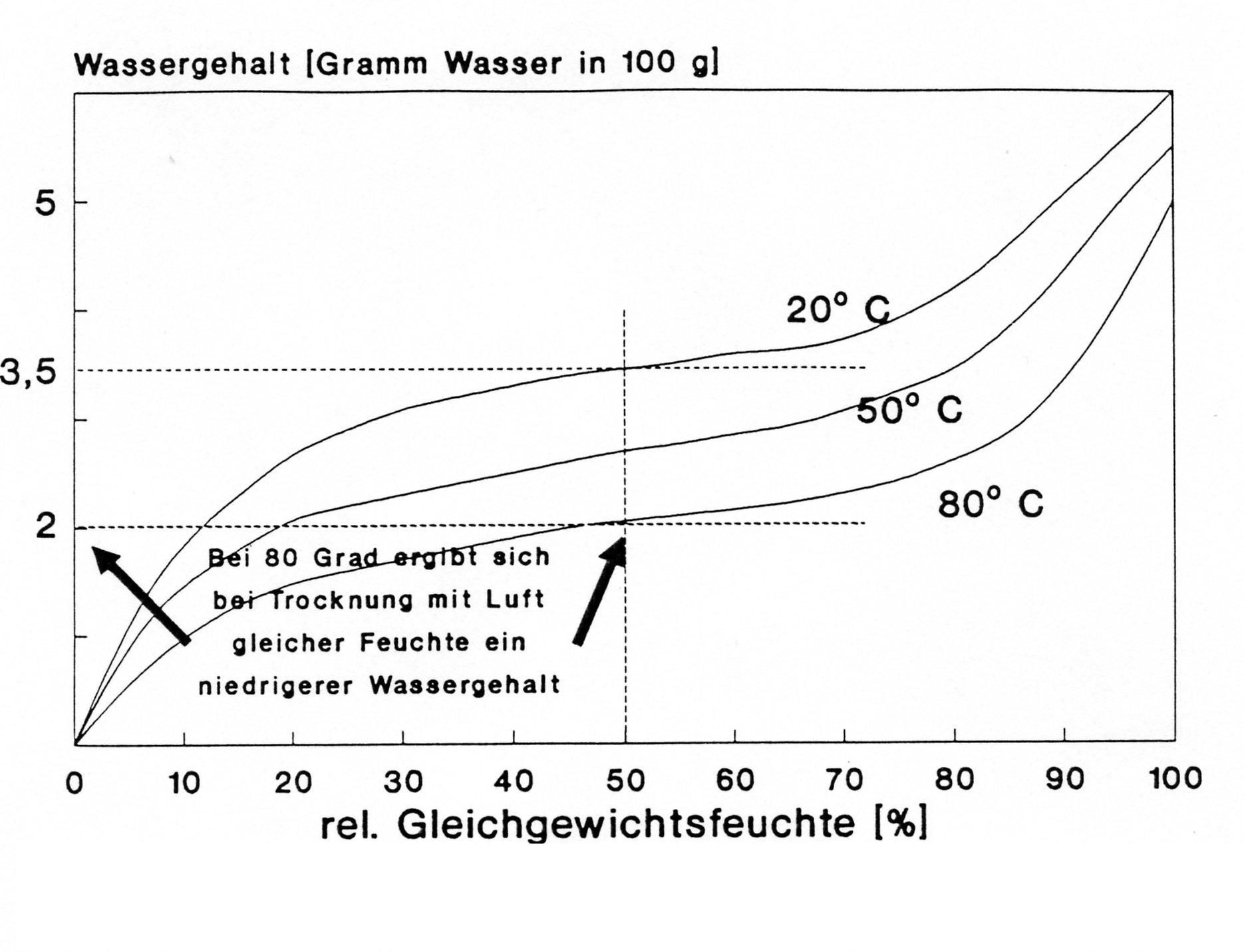
The figure shows the sorption isotherm of a sample at different temperatures. This sample is to be dried.
At a certain water content, the sample is completely saturated with water and can no longer absorb water. It has an equilibrium moisture content of 100% and even a RH of 99% dries this sample. If the water content decreases, the water remaining in the sample is more strongly bound. Therefore, to dry the sample further, a lower RH is required, for example, of 50% RH. According to the figure, the sample can only dry to 3.5g water content at 50% RH. Further water release is only possible if the RH decreases further or the temperature is increased. At 80°C, the sample will contain only 2g of water.
The described correlations apply to desorption, the release of water. Similar considerations apply to water uptake (absorption). Here the isotherm must be interpreted in such a way that at the humidity or temperature a certain humidification is possible. Absorption and desorption isotherms have the same course. The desorption isotherm, however, lies above the absorption isotherm. This is due to the fact that the water in the sample is bound with a certain amount of energy. This energy is released when the water is absorbed. For the same water content, water is absorbed more easily than it is released.
Coefficient of water absorption[edit]
A way of measuring the water absorption rate of a building material is the water absorption coefficient w with
Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle w= \frac {W[kg/m^2]}{\sqrt{t}[h^{0.5}]}}
with W = Amount of water in [kg/m²], t = time in [h]
That indicates how many kilograms of water are absorbed per hour from one square meter of surface by capillary suction.
| The water absorption coefficient w is a measurement showing the water absorption rates of building materials. |
The following classification of the water absorption coefficient is commonly used (data in kg/(m2*h0.5))[2][3]:
- absorbing (w > 2)
- water-repellent (w < 2)
- hydrophobic (w < 0.5)
- water impermeable (w < 0.001)
Water absorption can be measured with test tubes according to KARSTEN, MIROWSKI or PLEYERS.
Weblinks[edit]
Literature[edit]
There were no citations found in the article.