Salt crystallization in the Grotto Hall of the New Palace in Potsdam: Difference between revisions
No edit summary |
|||
| (7 intermediate revisions by 3 users not shown) | |||
| Line 2: | Line 2: | ||
English version by [[user:SLeithaeuser|Sandra Leithäuser]] | English version by [[user:SLeithaeuser|Sandra Leithäuser]] | ||
<br> | <br> | ||
back to [[Case Studies]] <br> | |||
<br> | |||
| Line 7: | Line 9: | ||
== The Grotto Hall in the New Palais, Potsdam == | == The Grotto Hall in the New Palais, Potsdam == | ||
The Grotto Hall is located | The Grotto Hall is located on the ground floor of the New Palais at the Sanssouci Palace Park, and was built between 1765-1769. Walls and ceilings are decorated with stucco (gypsum mortar) in which a variety of different materials such as minerals, rocks, fossils and shells are embedded presenting an exceptional collection of geological and natural history specimens. Even the floors use different stones to form patterns. | ||
Deterioration is evident on the materials, the most important corresponding to those located around the windows facing the park. In general, the intensity of the damage decreases towards the interior of the Palace. | |||
Preliminary investigations have shown that salt crystallization plays an important role in the deterioration processes of the Grotto Hall. Salt minerals crystallize on the walls and its subsurface, causing damage to the building materials. | |||
== Salt and indoor-climate in the Grotto Hall == | == Salt and indoor-climate in the Grotto Hall == | ||
To investigate the deterioration processes, specific reference areas were chosen and a method for monitoring their changes in combination with climate measurements was chosen <bib id="Laue:2002" />. It was not possible to take samples for quantitative salt analysis in the Grotto Hall, however,in efflorescence samples the two forms of sodium sulfate, thenardite [Na<sub>2</sub>SO<sub>4</sub>] and mirabilite [Na<sub>2</sub>SO<sub>4</sub>•10H<sub>2</sub>0] were found. A few samples also tested positive for gypsum, which could be from traces of the Grotto mortar and therefore not necessarily recrystallized. | |||
The sources of Na<sup>+</sup> salt ions are probably due | The sources of Na<sup>+</sup> salt ions are probably due from alkali containing Portland cement that was used to stabilize the building in previous interventions. These building materials contain alkali (e.g., Na<sup>+</sup>) that can react to form alkali carbonates. Under suitable moisture conditions these can react with other salts, for example the sulfate ions (SO<sub>4</sub><sup>2-</sup>)of the gypsum mortar, forming sodium sulfate <bib id="Arnold:1985b" />. | ||
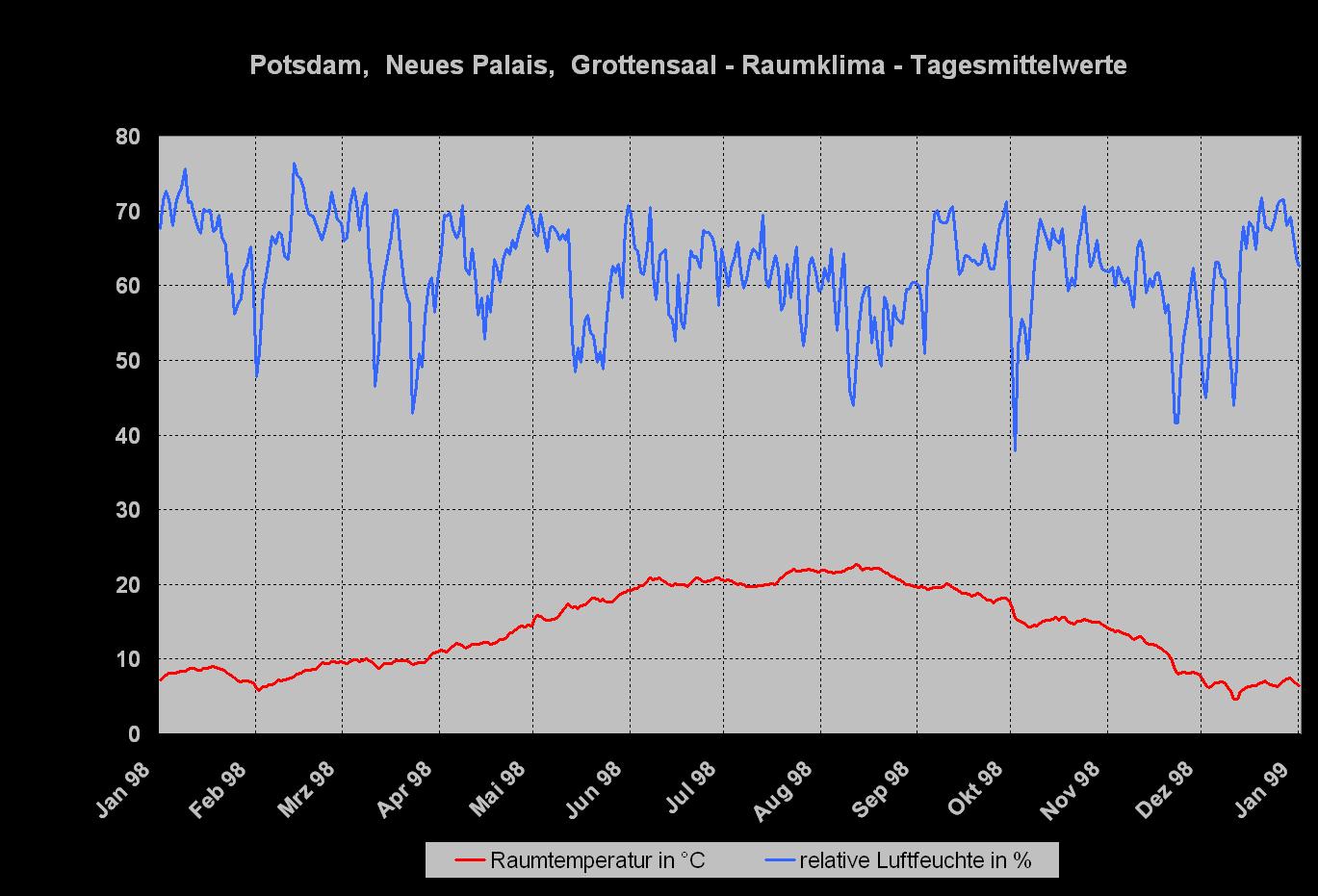
Figure 1 shows the daily average value of the air temperature and relative humidity in the Grotto Hall from January 1998 to January 1999. The climate curve is typical for an unheated room: The indoor-climate mirrors the outside climate in a subdued form. The temperature fluctuates between | Figure 1 shows the daily average value of the air temperature and relative humidity in the Grotto Hall from January 1998 to January 1999. The climate curve is typical for an unheated room: The indoor-climate mirrors the outside climate in a subdued form. The temperature fluctuates between 5°C and 20°C, the relative humidity varies mostly between 40% and 75%, only during a longer dry cold period in December did the relative humidity fall to values around 30%. | ||
<gallery perrow="1" heights="400px" widths="700px" caption="Indoor-climate in the Grotto Hall"> | <gallery perrow="1" heights="400px" widths="700px" caption="Indoor-climate in the Grotto Hall"> | ||
| Line 25: | Line 27: | ||
Climate measurements in combination with the observation of the reference areas showed that salt | Climate measurements in combination with the observation of the reference areas showed that salt crystallization of both sodium sulfate forms took place throughout the whole year. | ||
This | This can be explained as follows: The ion-rich solution inside the walls, consisting of moisture that was introduced by rising damp and water entering the external wall, evaporates at wall surface. As evaporation takes place, the salt solution becomes more saturated and crystallization of mirabillte takes place. | ||
If the relative humidity level sinks, mirabilite | If the relative humidity level sinks, mirabilite deliquesces turning into thenardite, depending on temperature and relative humidity. At increasing relative humidity levels re-hydration occurs and mirabilite may recrystalize(see <bib id="Steiger.etal:1998b" /> und <bib id="Laue:2002" />. | ||
For instance, the mineral thenardite crystallizes at a temperature of 20°C, below 76% RH, above 76% mirabilite crystallizes. The transformation process | For instance, the mineral thenardite crystallizes at a temperature of 20°C, below 76% RH, above 76% mirabilite crystallizes. The transformation process of thenardite into mirabilite is associated with an increase in volume of 314%, as described by Price and Brimblecombe <bib id="Price.etal:1994" />. Every time the relative humidity reaches the critical level, where the phase change respectively takes place, the re-crystallization processes lead to deterioration. | ||
Since the temperature as well as the relative humidity in the Grotto Hall fluctuates all the time, the critical climate values are often exceeded, | Since the temperature as well as the relative humidity in the Grotto Hall fluctuates all the time, the critical climate values are often exceeded, inducing the observed damage. | ||
== Sequence of damage processes == | == Sequence of damage processes == | ||
| Line 39: | Line 41: | ||
<biblist /> | <biblist /> | ||
[[category:Laue,Steffen]][[ | [[category:Laue,Steffen]][[Category:R-GGrassegger]][[category:Potsdam_Grottensaal]][[category:approved]] | ||
Latest revision as of 09:51, 10 January 2015
Author: Steffen Laue
English version by Sandra Leithäuser
back to Case Studies
The Grotto Hall in the New Palais, Potsdam[edit]
The Grotto Hall is located on the ground floor of the New Palais at the Sanssouci Palace Park, and was built between 1765-1769. Walls and ceilings are decorated with stucco (gypsum mortar) in which a variety of different materials such as minerals, rocks, fossils and shells are embedded presenting an exceptional collection of geological and natural history specimens. Even the floors use different stones to form patterns. Deterioration is evident on the materials, the most important corresponding to those located around the windows facing the park. In general, the intensity of the damage decreases towards the interior of the Palace. Preliminary investigations have shown that salt crystallization plays an important role in the deterioration processes of the Grotto Hall. Salt minerals crystallize on the walls and its subsurface, causing damage to the building materials.
Salt and indoor-climate in the Grotto Hall[edit]
To investigate the deterioration processes, specific reference areas were chosen and a method for monitoring their changes in combination with climate measurements was chosen [Laue:2002]Title: Verwitterung von Naturstein durch lösliche Salze an wechselfeuchter Luft
Author: Laue, Steffen . It was not possible to take samples for quantitative salt analysis in the Grotto Hall, however,in efflorescence samples the two forms of sodium sulfate, thenardite [Na2SO4] and mirabilite [Na2SO4•10H20] were found. A few samples also tested positive for gypsum, which could be from traces of the Grotto mortar and therefore not necessarily recrystallized.
The sources of Na+ salt ions are probably due from alkali containing Portland cement that was used to stabilize the building in previous interventions. These building materials contain alkali (e.g., Na+) that can react to form alkali carbonates. Under suitable moisture conditions these can react with other salts, for example the sulfate ions (SO42-)of the gypsum mortar, forming sodium sulfate [Arnold:1985b]Title: Moderne alkalische Baustoffe und die Probleme bei der Konservierung von Baudenkmälern
. It was not possible to take samples for quantitative salt analysis in the Grotto Hall, however,in efflorescence samples the two forms of sodium sulfate, thenardite [Na2SO4] and mirabilite [Na2SO4•10H20] were found. A few samples also tested positive for gypsum, which could be from traces of the Grotto mortar and therefore not necessarily recrystallized.
The sources of Na+ salt ions are probably due from alkali containing Portland cement that was used to stabilize the building in previous interventions. These building materials contain alkali (e.g., Na+) that can react to form alkali carbonates. Under suitable moisture conditions these can react with other salts, for example the sulfate ions (SO42-)of the gypsum mortar, forming sodium sulfate [Arnold:1985b]Title: Moderne alkalische Baustoffe und die Probleme bei der Konservierung von Baudenkmälern
Author: Arnold, Andreas .
.
Figure 1 shows the daily average value of the air temperature and relative humidity in the Grotto Hall from January 1998 to January 1999. The climate curve is typical for an unheated room: The indoor-climate mirrors the outside climate in a subdued form. The temperature fluctuates between 5°C and 20°C, the relative humidity varies mostly between 40% and 75%, only during a longer dry cold period in December did the relative humidity fall to values around 30%.
- Indoor-climate in the Grotto Hall
Climate measurements in combination with the observation of the reference areas showed that salt crystallization of both sodium sulfate forms took place throughout the whole year.
This can be explained as follows: The ion-rich solution inside the walls, consisting of moisture that was introduced by rising damp and water entering the external wall, evaporates at wall surface. As evaporation takes place, the salt solution becomes more saturated and crystallization of mirabillte takes place.
If the relative humidity level sinks, mirabilite deliquesces turning into thenardite, depending on temperature and relative humidity. At increasing relative humidity levels re-hydration occurs and mirabilite may recrystalize(see [Steiger.etal:1998b]Title: Bedingungen für die Kristallisation verschiedener Salzhydrate am Beispiel Thenardit/Mirabilit
Author: Steiger, Michael; Dannecker, Walter und [Laue:2002]Title: Verwitterung von Naturstein durch lösliche Salze an wechselfeuchter Luft
und [Laue:2002]Title: Verwitterung von Naturstein durch lösliche Salze an wechselfeuchter Luft
Author: Laue, Steffen .
For instance, the mineral thenardite crystallizes at a temperature of 20°C, below 76% RH, above 76% mirabilite crystallizes. The transformation process of thenardite into mirabilite is associated with an increase in volume of 314%, as described by Price and Brimblecombe [Price.etal:1994]Title: Preventing salt damage in porous materials
.
For instance, the mineral thenardite crystallizes at a temperature of 20°C, below 76% RH, above 76% mirabilite crystallizes. The transformation process of thenardite into mirabilite is associated with an increase in volume of 314%, as described by Price and Brimblecombe [Price.etal:1994]Title: Preventing salt damage in porous materials
Author: Price, Clifford A.; Brimblecomb, Peter . Every time the relative humidity reaches the critical level, where the phase change respectively takes place, the re-crystallization processes lead to deterioration.
Since the temperature as well as the relative humidity in the Grotto Hall fluctuates all the time, the critical climate values are often exceeded, inducing the observed damage.
. Every time the relative humidity reaches the critical level, where the phase change respectively takes place, the re-crystallization processes lead to deterioration.
Since the temperature as well as the relative humidity in the Grotto Hall fluctuates all the time, the critical climate values are often exceeded, inducing the observed damage.
Sequence of damage processes[edit]
The sequence of damage processes was detected for the Grotto Hall and can be described as follows: due to moisture in the substrate and surface water (splash back), moisture enters the masonry walls. When the masonry solution evaporates, the salt solution becomes more saturated and the salts start crystallizing to become mirabilite (at the determined temperature ranges in the Grotto Hall). When the climate is dry – between approx. 60% and 75% RH and in dependence of temperature- the dehydration of mirabilite takes place [Na2SO4•10H20], transforming into thenardite [Na2SO4]. Mirabilite is unstable in these humidity conditions and looses its water of crystallization, then thenardite crystallizes. At increasing relative humidity levels, the hydration from thenardite to mirabilite occurs. This crystallization process is accompanied by an increase in volume. While hydration and dehydration processes occur, probably the break down of one crystal system is rapidly followed by the crystallization of the other hydration stage. The hydration and dehydration effect, leads to continuous re-crystallization of the salts. The previously described damages are due to the associated increase and decrease in volume.
Literature[edit]
| [Arnold:1985b] | Arnold, Andreas (1985): Moderne alkalische Baustoffe und die Probleme bei der Konservierung von Baudenkmälern. In: Snethlage, Rolf (eds.): Natursteinkonservierung, Internationales Kolloquium, München, Bayer. Landesamt für Denkmalpflege, 152-161. |  |
| [Laue:2002] | Laue, Steffen (2002): Verwitterung von Naturstein durch lösliche Salze an wechselfeuchter Luft. In: Institut für Steinkonservierung e.V. (IFS) (eds.): IFS-Tagung 2002: Salze im historischen Natursteinmauerwerk, IFS, Mainz, 19-30. |   |
| [Price.etal:1994] | Price, Clifford A.; Brimblecomb, Peter (1994): Preventing salt damage in porous materials. In:: Preventive conservation: practice, theory and research. Preprints of the contributions to the Ottawa Congress, 12-16 September 1994, International Institute for Conservation of Historic and Artistic Works, 90-93. |  |
| [Steiger.etal:1998b] | Steiger, Michael; Dannecker, Walter (1998): Bedingungen für die Kristallisation verschiedener Salzhydrate am Beispiel Thenardit/Mirabilit, interner Bericht, 123-133. |  |