Sodium sulfate heptahydrate: Difference between revisions
| (2 intermediate revisions by 2 users not shown) | |||
| Line 67: | Line 67: | ||
==The importance of the heptahydrate in the damage process== | ==The importance of the heptahydrate in the damage process== | ||
For more informations see <bib id="Saidov: | For more informations see <bib id="Saidov:2012"/> | ||
==Weblinks== | ==Weblinks== | ||
Latest revision as of 08:27, 12 April 2023
Author: Amelie Stahlbuhk
back to Sulfate
| Sodium sulfate heptahydrate | |
| Mineralogical name | |
| Chemical name | sodium sulfate heptahydrate |
| Trivial name | |
| Chemical formula | Na2SO4•7H2O |
| Other forms | Na2SO4•10H2O (Mirabilite) |
| Crystal system | |
| Crystal structure | |
| Deliquescence humidity 20°C | 89.1 % |
| Solubility (g/l) at 20°C | 3.143 mol/kg |
| Density (g/cm³) | |
| Molar volume | |
| Molar weight | 268,14 g/mol |
| Transparency | |
| Cleavage | |
| Crystal habit | |
| Twinning | |
| Phase transition | |
| Chemical behavior | |
| Comments | |
| Crystal Optics | |
| Refractive Indices | |
| Birefringence | |
| Optical Orientation | |
| Pleochroism | |
| Dispersion | |
| Used Literature | |
| [Steiger.etal:2008]Title: Crystallization of sodium sulfate phases in porous materials: The phase diagram Na2SO4–H2O and the generation of stress Author: Steiger, Michael; Asmussen, Sönke 
| |
Introduction[edit]
Sodium sulfate heptahydrate is a metastable phase of sodium sulfate. Its formation can be observed during the rapid cooling of a solution that is saturated at 40 °C [Gans:1978]Title: Thermodynamic stability of sodium sulfate heptahydrate
Author: Gans, W.
Solubility[edit]
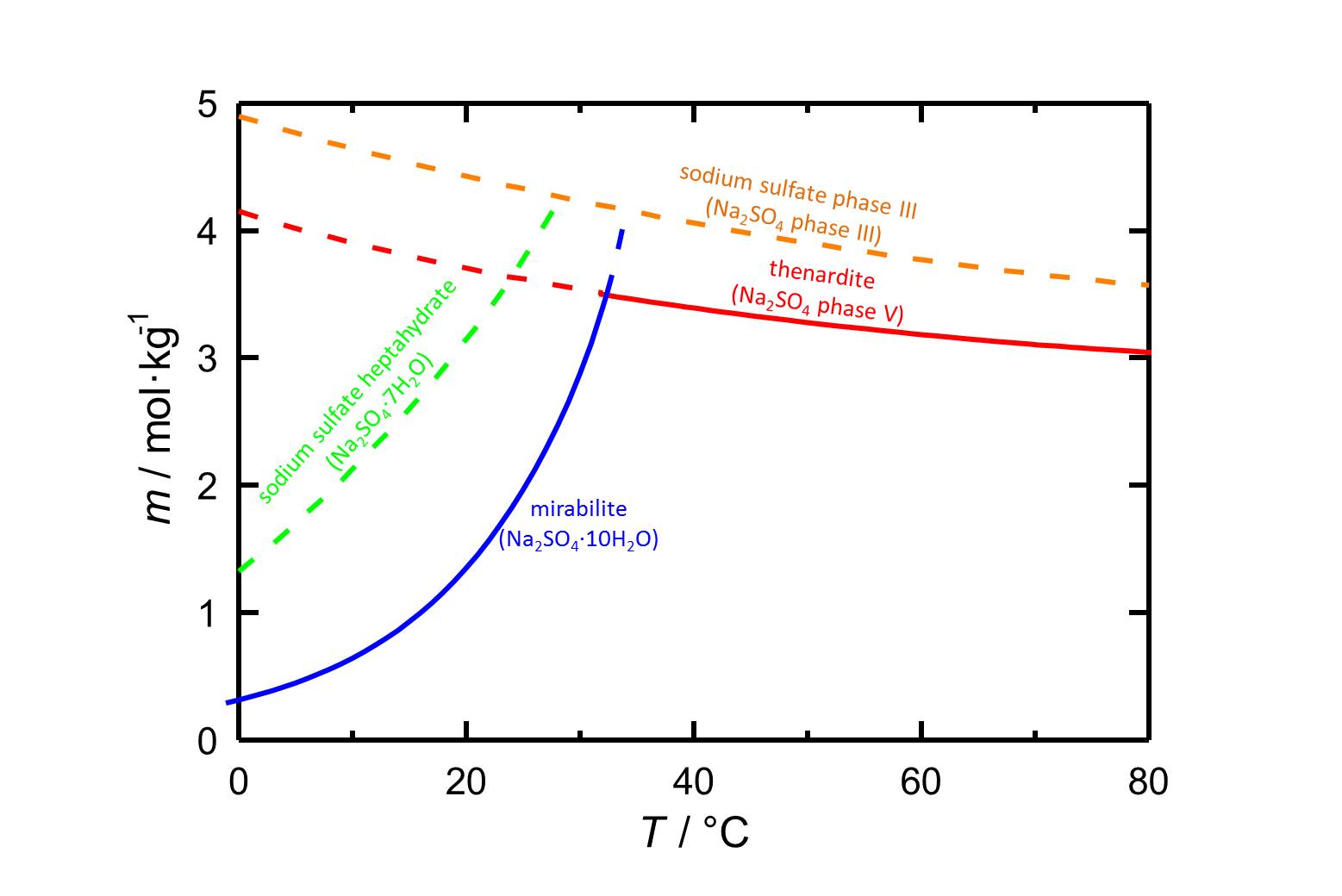
Author: Steiger, Michael; Asmussen, Sönke

The solubility of the heptahydrate at 20 °C is 3.145 mol/kg [Steiger.etal:2008]Title: Crystallization of sodium sulfate phases in porous materials: The phase diagram Na2SO4–H2O and the generation of stress
Author: Steiger, Michael; Asmussen, Sönke . Figure 1 indicates that, eventhough it is a metastable phase, the heptahydrate is more relevant at lower temperatures.
. Figure 1 indicates that, eventhough it is a metastable phase, the heptahydrate is more relevant at lower temperatures.
Hygroscopicity[edit]
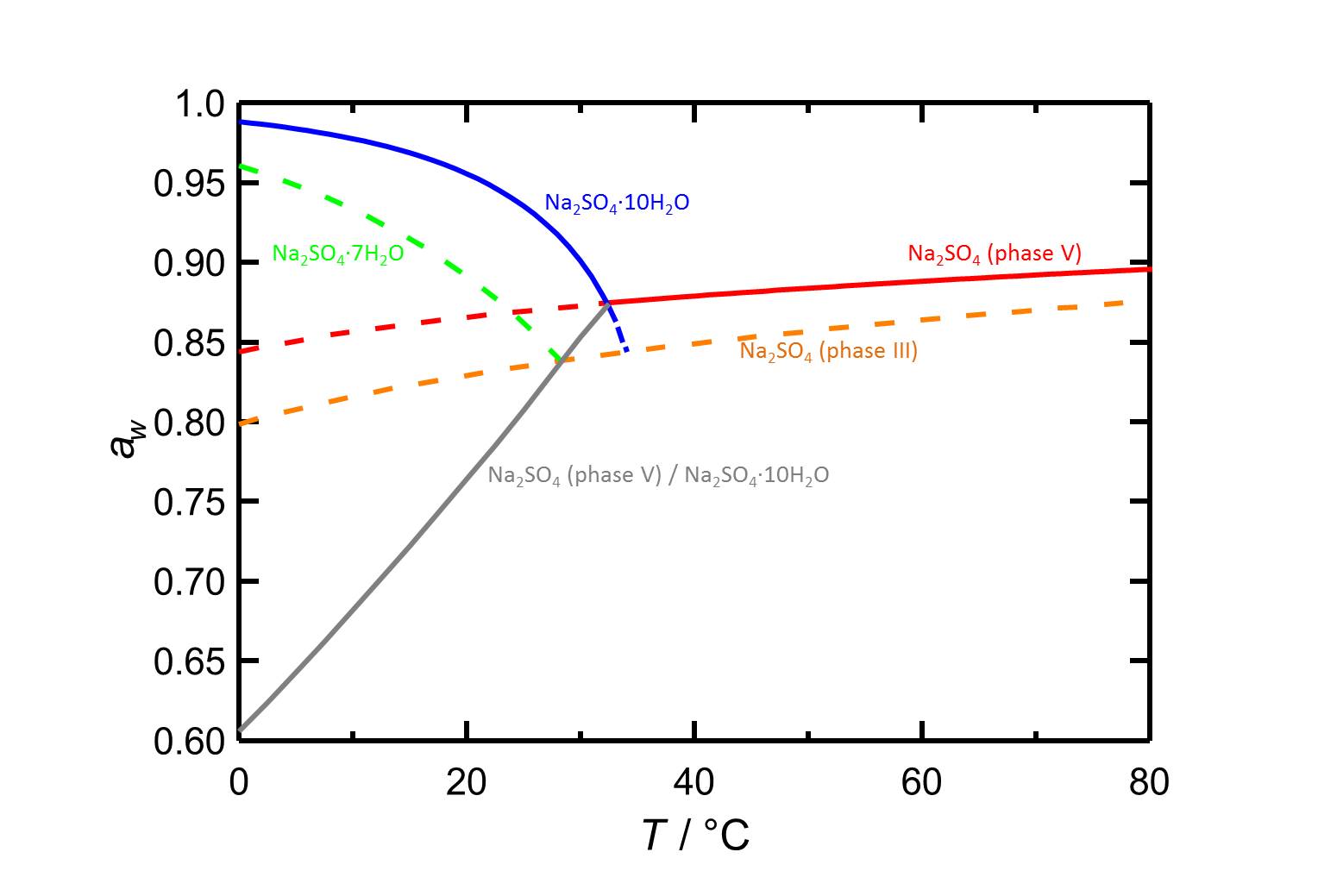
Author: Steiger, Michael; Asmussen, Sönke

At 20 °C the deliquescence humidity lies at 89.1 %. The values are higher at lower temperatures (table 1).
| 0°C | 10°C | 20°C |
| 96.1%r.h. | 93.3%r.h. | 89.1%r.h. |
The importance of the heptahydrate in the damage process[edit]
For more informations see [Saidov:2012]Title: Sodium sulfate heptahydrate in weathering phenomena of porous materials
Author: Saidov, Tamerlan Adamovich
Weblinks[edit]
Literature[edit]
| [Gans:1978] | Gans, W. (1978): Thermodynamic stability of sodium sulfate heptahydrate. In: Zeitschrift für Physikalische Chemie, 111 (1), 39-46, Url |  |
| [Saidov:2012] | Saidov, Tamerlan Adamovich (2012): Sodium sulfate heptahydrate in weathering phenomena of porous materials. dissertation, Technische Universiteit Eindhoven, Url |  |
| [Steiger.etal:2008] | Steiger, Michael; Asmussen, Sönke (2008): Crystallization of sodium sulfate phases in porous materials: The phase diagram Na2SO4–H2O and the generation of stress. In: Geochimica et Cosmochimica Acta, 72 (17), 4291-4306, Url |  |