Halite: Difference between revisions
No edit summary |
|||
| (49 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
Authors: [[User:Hschwarz|Hans-Jürgen Schwarz]], [[user:NMainusch|Nils Mainusch]] | |||
Authors: [[User:Hschwarz|Hans-Jürgen Schwarz]], Nils Mainusch | <br>English version by [[user:CGerdwilker|Christa Gerdwilker]] | ||
<br>English version by Christa Gerdwilker | |||
<br>back to [[Chloride]] | <br>back to [[Chloride]] | ||
{{Infobox_Salt | {{Infobox_Salt | ||
|Footnote=<ref>http://webmineral.com/data/Halite.shtml accessed 28.07.2010</ref><ref>http://www.mindat.org/min-1804.html accessed 28.07.2010</ref><ref>http://www.mineralienatlas.de/lexikon/index.php/MineralData?mineral=Halit accessed 28.07.2010</ref> | |Footnote=<ref>http://webmineral.com/data/Halite.shtml accessed 28.07.2010</ref><ref>http://www.mindat.org/min-1804.html accessed 28.07.2010</ref><ref>http://www.mineralienatlas.de/lexikon/index.php/MineralData?mineral=Halit accessed 28.07.2010</ref> <ref name=hydrohalit>http://www.mineralienatlas.de/lexikon/index.php/MineralData?mineral=Hydrohalit accessed 28.07.2010</ref> | ||
|photo = [[Image:NaCl 27.4.2006-10x.JPG|300px]] | |photo = [[Image:NaCl 27.4.2006-10x.JPG|300px]] | ||
|mineralogical_Name = Halite | |mineralogical_Name = Halite | ||
| Line 12: | Line 11: | ||
|Trivial_Name = Common Salt, Rock Salt | |Trivial_Name = Common Salt, Rock Salt | ||
|chemical_Formula = NaCl | |chemical_Formula = NaCl | ||
|Hydratforms= | |Hydratforms=NaCl•2H<sub>2</sub>O ([[Hydrohalite]]) | ||
|Crystal_System =cubic | |Crystal_System =cubic | ||
|Crystal_Structure = | |Crystal_Structure = | ||
|Deliqueszenzhumidity =75. | |Deliqueszenzhumidity =75.4% | ||
|Solubility = | |Solubility = 6.135 mol/kg | ||
|Density =2.163 g/cm<sup>3</sup> | |Density =2.163 g/cm<sup>3</sup> | ||
|MolVolume =27.02 cm<sup>3</sup>/mol | |MolVolume =27.02 cm<sup>3</sup>/mol | ||
| Line 24: | Line 23: | ||
|Crystal_Habit =cubic crystal, granular, massive aggregates | |Crystal_Habit =cubic crystal, granular, massive aggregates | ||
|Twinning =none | |Twinning =none | ||
|Refractive_Indices =n=1. | |Refractive_Indices =n<sub>D</sub>=1.5443 | ||
|Birefringence = | |Birefringence = | ||
|optical_Orientation =isotropic | |optical_Orientation =isotropic | ||
| Line 31: | Line 30: | ||
|Phase_Transition = | |Phase_Transition = | ||
|chemBehavior = | |chemBehavior = | ||
|Comments = | |Comments = water soluble | ||
|Literature =<bib id="Steiger.etal:2014"/> <bib id="Robie.etal:1978"/> <bib id="Dana:1951"/> | |||
}} | }} | ||
== Abstract == | == Abstract == | ||
== | == Occurrence == | ||
Sodium chloride is obtained through mining or derived from the sea or salt lakes and is commonly used for cooking or as | Sodium chloride is obtained through mining or derived from the sea or salt lakes and is commonly used for cooking or as deicing salt for roads. <br> | ||
The | The sodium chloride content of sea water is around 2.7 M.%.<br> | ||
== | == Origin of the halite found on monuments == | ||
Sodium chloride can enter buildings or monuments when these are in contact with materials containing this salt or even other salts containing either sodium or chloride, that might combine to produce NaCl in or efflorescence on them. Contamination with sodium and chloride ions can also occur through contact with salt laden ground or surface water. A range of cleaning materials (e.g., acidic and alkaline cleaners or combination of them), or previously used restoration materials (e.g., water glass) as well as Portland cement, can introduce sodium and chloride ions into monuments. Further common and important sources are deicing salts and maritime environments where the air and fogs may contain a significant amount of sodium chloride in suspension or dissolved in droplets.<br> | |||
<br clear=all> | <br clear=all> | ||
| Line 48: | Line 48: | ||
== Solubility behavior == | == Solubility behavior == | ||
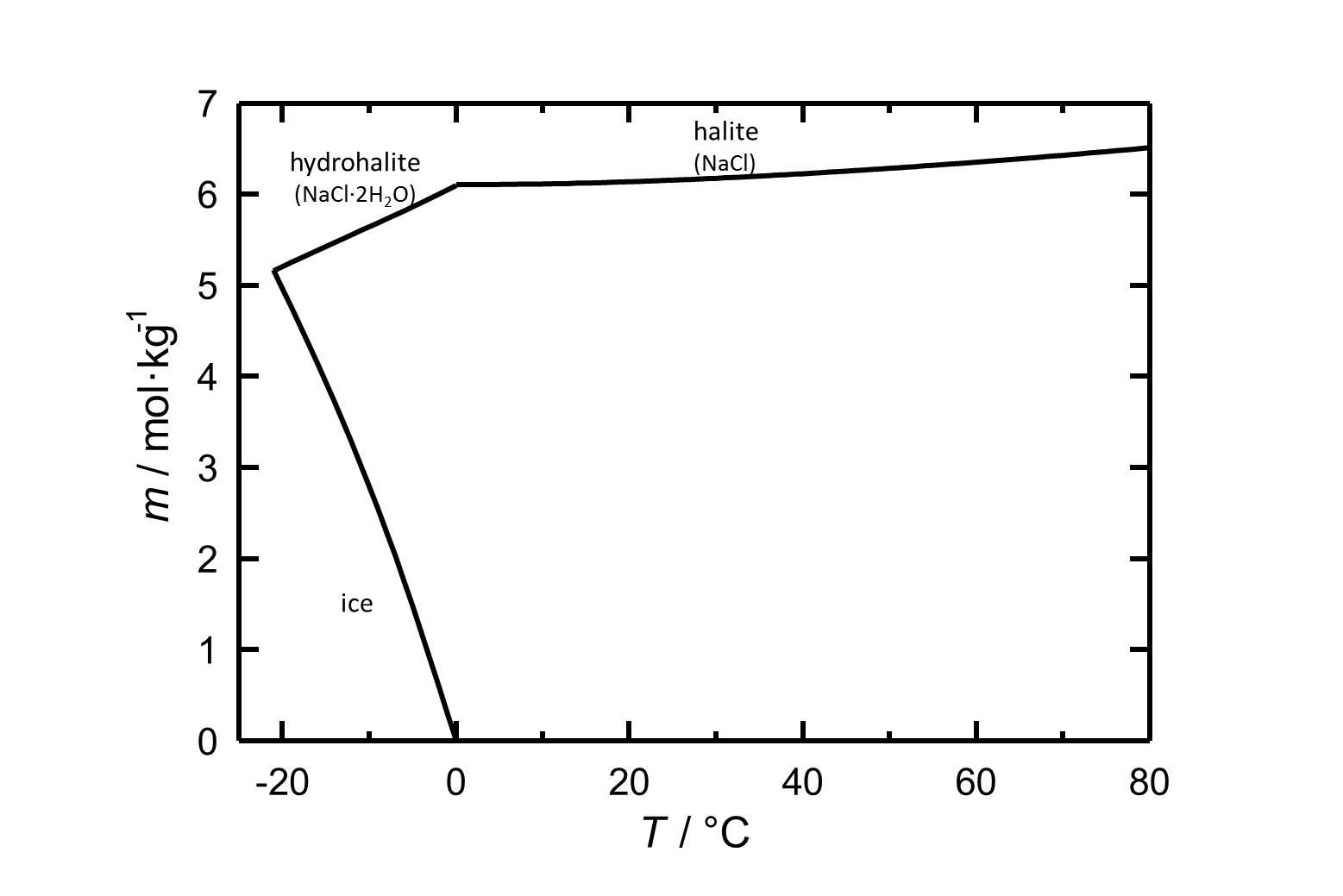
The commonly occurring halite | The commonly occurring halite has a solubility of 6.13 mol/kg (20°C) and can be considered as a very soluble and, therefore, easily mobilized salt. Its solubility changes not significantly within a temperature range of 10 -30°C. | ||
[[Image:NaCl | [[Image:S NaCl.jpg|thumb|left|800px|Figure 1: Solubility of sodium chloride in water. The molality ''m'' [n(NaCl)•kg(H<sub>2</sub>O)<sup>-1</sup>] is plotted versus the temperature.]] | ||
<br clear="all"> | <br clear="all"> | ||
{|border="2" cellspacing="0" cellpadding="4" width="52%" align="left" class="wikitable" | {|border="2" cellspacing="0" cellpadding="4" width="52%" align="left" class="wikitable" | ||
|+''Table 1: Solubility of halite | |+''Table 1: Solubility of halite at different round temperatures [according to <bib id="Steiger.etal:2008c"/>.'' | ||
|- | |- | ||
|bgcolor = "#F0F0F0"| '''Temperature''' | |bgcolor = "#F0F0F0"| '''Temperature''' | ||
|bgcolor = "#F0F0F0" align=center| '''10°C''' | |bgcolor = "#F0F0F0" align=center| '''10°C''' | ||
|bgcolor = "#F0F0F0" align=center| '''20°C''' | |bgcolor = "#F0F0F0" align=center| '''20°C''' | ||
|bgcolor = "#F0F0F0" align=center| '''30°C''' | |||
|bgcolor = "#F0F0F0" align=center| '''40°C''' | |bgcolor = "#F0F0F0" align=center| '''40°C''' | ||
|- | |- | ||
|bgcolor = "#F7F7F7" | Solubility [ | |bgcolor = "#F7F7F7" | Solubility [mol/kg] | ||
|bgcolor = "#FFFFEO" align=center| | |bgcolor = "#FFFFEO" align=center| 6.11 | ||
|bgcolor = "#FFFFEO" align=center| | |bgcolor = "#FFFFEO" align=center| 6.13 | ||
|bgcolor = "#FFFFEO" align=center| | |bgcolor = "#FFFFEO" align=center| 6.17 | ||
|bgcolor = "#FFFFEO" align=center| 6.22 | |||
|} | |} | ||
<br clear=all> | <br clear=all> | ||
== Hygroscopicity == | == Hygroscopicity == | ||
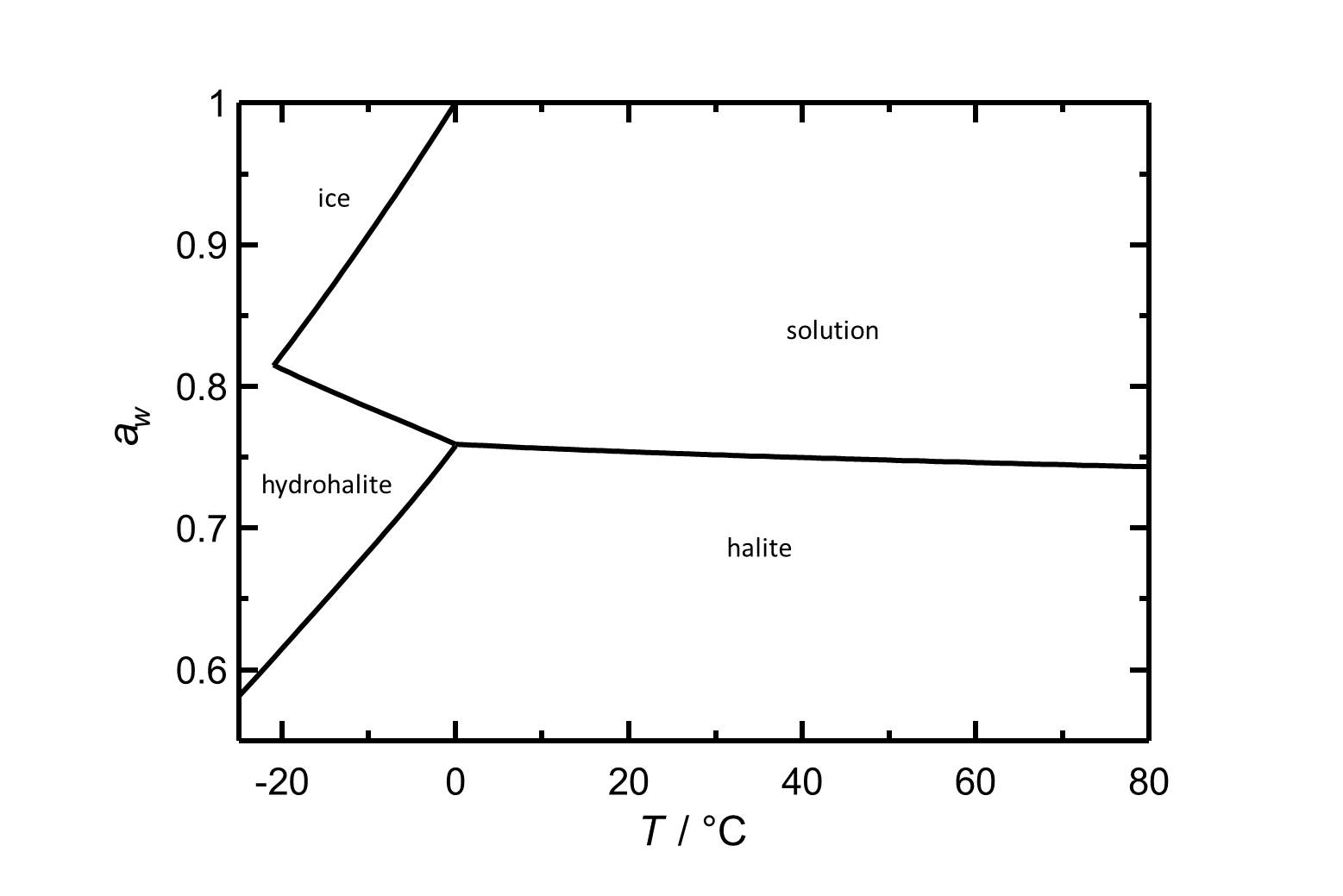
Halite has a deliquescence humidity of about 75% RH, therefore it tends to pick up moisture easily in most temperate climates. | |||
[[Image:D NaCl e.jpg|thumb|left|800px|Figure 2: Deliquescence behaviour of sodium chloride. The water activity ''a<sub>w</sub>'' is plotted versus the temperature.]] | |||
<!--Temperature variations hardly affect the deliquescence point of halite which is illustrated below in comparison with potassium nitrate and natrite.--> | |||
<br clear=all> | |||
{|border="2" cellspacing="0" cellpadding="4" width="52%" align="left" class="wikitable" | |||
|+''Table 2: Deliquescence humidities of sodium chloride at different round temperatures <bib id="Steiger etal: 2014"/>'' | |||
|- | |||
|bgcolor = "#F0F0F0" align=center| 0°C | |||
|bgcolor = "#F0F0F0" align=center| 10°C | |||
|bgcolor = "#F0F0F0" align=center| 20°C | |||
|bgcolor = "#F0F0F0" align=center| 30°C | |||
|bgcolor = "#F0F0F0" align=center| 40°C | |||
|bgcolor = "#F0F0F0" align=center| 50°C | |||
|- | |||
|bgcolor = "#FFFFEO" align=center| 75.9%r.h. | |||
|bgcolor = "#FFFFEO" align=center| 75.6%r.h. | |||
|bgcolor = "#FFFFEO" align=center| 75.4%r.h. | |||
|bgcolor = "#FFFFEO" align=center| 75.2%r.h. | |||
|bgcolor = "#FFFFEO" align=center| 75.2%r.h. | |||
|bgcolor = "#FFFFEO" align=center| 74.8%r.h. | |||
|} | |||
<br clear=all> | |||
<br> '''Moisture sorption:'''<br>Theoretically 1g NaCl | <br> '''Moisture sorption:'''<br>Theoretically 1g NaCl can take up 4.3g of moisture, i.e., water vapor. The moisture sorption during varying relative humidity levels is: | ||
<br clear="all"> | <br clear="all"> | ||
{|border="2" cellspacing="0" cellpadding="4" width="52%" align="left" class="wikitable sortable" | {|border="2" cellspacing="0" cellpadding="4" width="52%" align="left" class="wikitable sortable" | ||
|+'' | |+''Table 3:Moisture sorption in M% after 56 days according to <bib id=''Vogt.etal:1993''/> | ||
|- | |- | ||
|bgcolor = "#F0F0F0"| '''Relative humidity during storge/salt phase''' | |bgcolor = "#F0F0F0" align=center| '''Relative humidity during storge/salt phase''' | ||
|bgcolor = "#F0F0F0" align=center| '''NaCl''' | |bgcolor = "#F0F0F0" align=center| '''NaCl''' | ||
|- | |- | ||
|bgcolor = "#F7F7F7" | '''87% RH''' | |bgcolor = "#F7F7F7" align=center| '''87% RH''' | ||
|bgcolor = "#FFFFEO" align=center| 153 | |bgcolor = "#FFFFEO" align=center| 153 | ||
|- | |- | ||
|bgcolor = "#F7F7F7" | '''81% RH''' | |bgcolor = "#F7F7F7" align=center| '''81% RH''' | ||
|bgcolor = "#FFFFEO" align=center| 22 | |bgcolor = "#FFFFEO" align=center| 22 | ||
|- | |- | ||
|bgcolor = "#F7F7F7" | '''79% RH''' | |bgcolor = "#F7F7F7" align=center| '''79% RH''' | ||
|bgcolor = "#FFFFEO" align=center| 7 | |bgcolor = "#FFFFEO" align=center| 7 | ||
|} | |} | ||
<br clear=all> | <br clear=all> | ||
<!-- | |||
== Crystallization pressure == | == Crystallization pressure == | ||
The crystallization of halite from | The crystallization of halite from an aqueous solution results in a crystallization pressure of 55,4-65,4 N/mm<sup>2</sup> <bib id="Winkler:1975" /> (for comparison, the crystallization pressure of different salts can range from 7,2-65,4 N/mm<sup>2</sup>). These values need to be considered in conjunction with temperature and concentration and can, therefore, only act as indicators of damage potential in relation to salt crystallization pressure. In comparison to other salts, the crystallization pressure of halite is extremely high. | ||
--> | |||
== Hydration behavior == | == Hydration behavior == | ||
Under normal conditions only | Under normal environmental conditions only halite will crystallize out of a saturated solution. The hydrated form, dihydrate hydrohalite<ref name=hydrohalit>http://www.mineralienatlas.de/lexikon/index.php/MineralData?mineral=Hydrohalit accessed 28.07.2010</ref> will only precipitate out at temperatures below 0.15°C. | ||
<!-- | <!-- | ||
=== Hydration pressure === | === Hydration pressure === | ||
=== | === Transition reactions === | ||
== <br>Analytical validation == | == <br>Analytical validation == | ||
| Line 115: | Line 140: | ||
== Microscopy == | == Microscopy == | ||
=== Laboratory | === Laboratory analysis === | ||
Sodium chloride crystals can be reliably identified on the basis of morphological features. Individual particles usually form cubic or octahedral shapes and, therefore, clearly display right angles in their crystal construction. <br> | Sodium chloride crystals can be reliably identified on the basis of their morphological features. Individual particles usually form cubic or octahedral shapes and, therefore, clearly display right angles in their crystal construction. <br> | ||
'''Refractive index:''' n<sub>D</sub> = 1 | '''Refractive index:''' n<sub>D</sub> = 1.544<br>'''Crystal category:''' cubic<br> | ||
''' | '''Examination by polarized microscopy:''' | ||
There are few salts belonging to the cubic crystal system which can be found in masonry, i.e., sodium chloride (halite), potassium chloride (sylvite) and calcium fluoride (flourite). Only the two first salts are highly soluble and therefore they are the ones that can cause damage to the masonry. Because of its isotropic internal structure these salts do not display birefringence. | |||
The | The refractive index can be measured with the immersion method by using a standard oil with a refractive index of n<sub>D</sub> =1.518. Halite crystals display the same optical density in every direction so that the speed and orientation of linear polarized light waves are not distorted, therefore, when viewed between crossed polars, the crystals are not visible, i.e., they show "extinction". | ||
<br> ''' | <br> '''Differentiation of halite from similar salts:'''<br> | ||
The | The three above mentioned isotropic salts can be easily differentiated. | ||
<br clear="all"> | <br clear="all"> | ||
{|border="2" cellspacing="0" cellpadding="4" width="50%" align="left" class="wikitable" | {|border="2" cellspacing="0" cellpadding="4" width="50%" align="left" class="wikitable" | ||
|+''Table | |+''Table 4: Identification features of other chlorides'' | ||
|- | |- | ||
{| cellspacing="1" cellpadding="1" border="1" style="width: 498px; height: 85px;" | {| cellspacing="1" cellpadding="1" border="1" style="width: 498px; height: 85px;" | ||
| Line 141: | Line 166: | ||
|- | |- | ||
|bgcolor = "#F7F7F7"| [[Sylvine]] KCl | |bgcolor = "#F7F7F7"| [[Sylvine]] KCl | ||
|bgcolor = "#FFFFEO"| Refractive index | |bgcolor = "#FFFFEO"| Refractive index below 1,518. | ||
|- | |- | ||
|bgcolor = "#F7F7F7"| [[Fluorite]] CaF<sub>2</sub> | |bgcolor = "#F7F7F7"| [[Fluorite]] CaF<sub>2</sub> | ||
|bgcolor = "#FFFFEO"| Refractive index | |bgcolor = "#FFFFEO"| Refractive index below 1,518, barely water soluble. | ||
|} | |} | ||
| Line 174: | Line 199: | ||
=== Under the polarizing microscope === | === Under the polarizing microscope === | ||
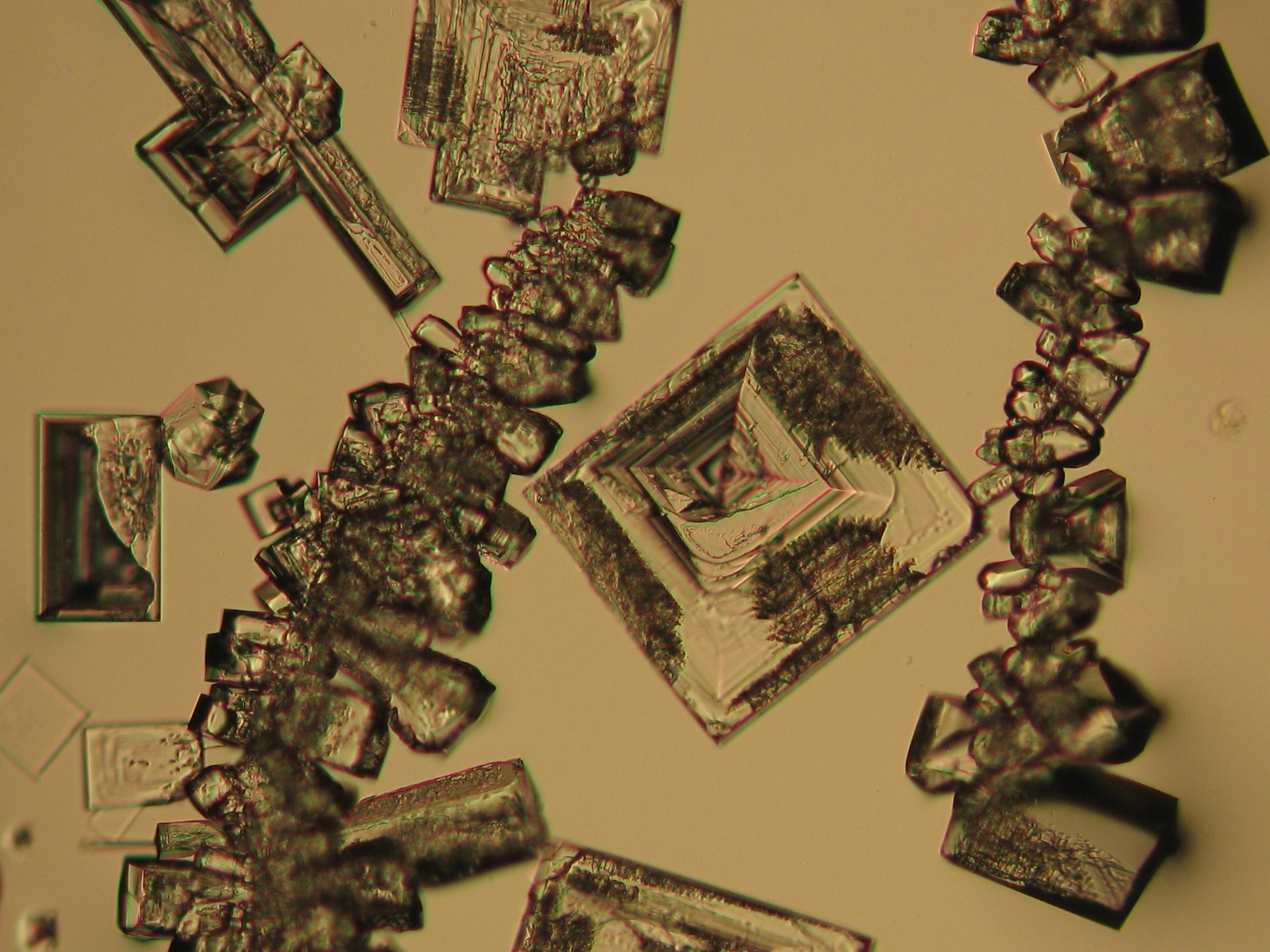
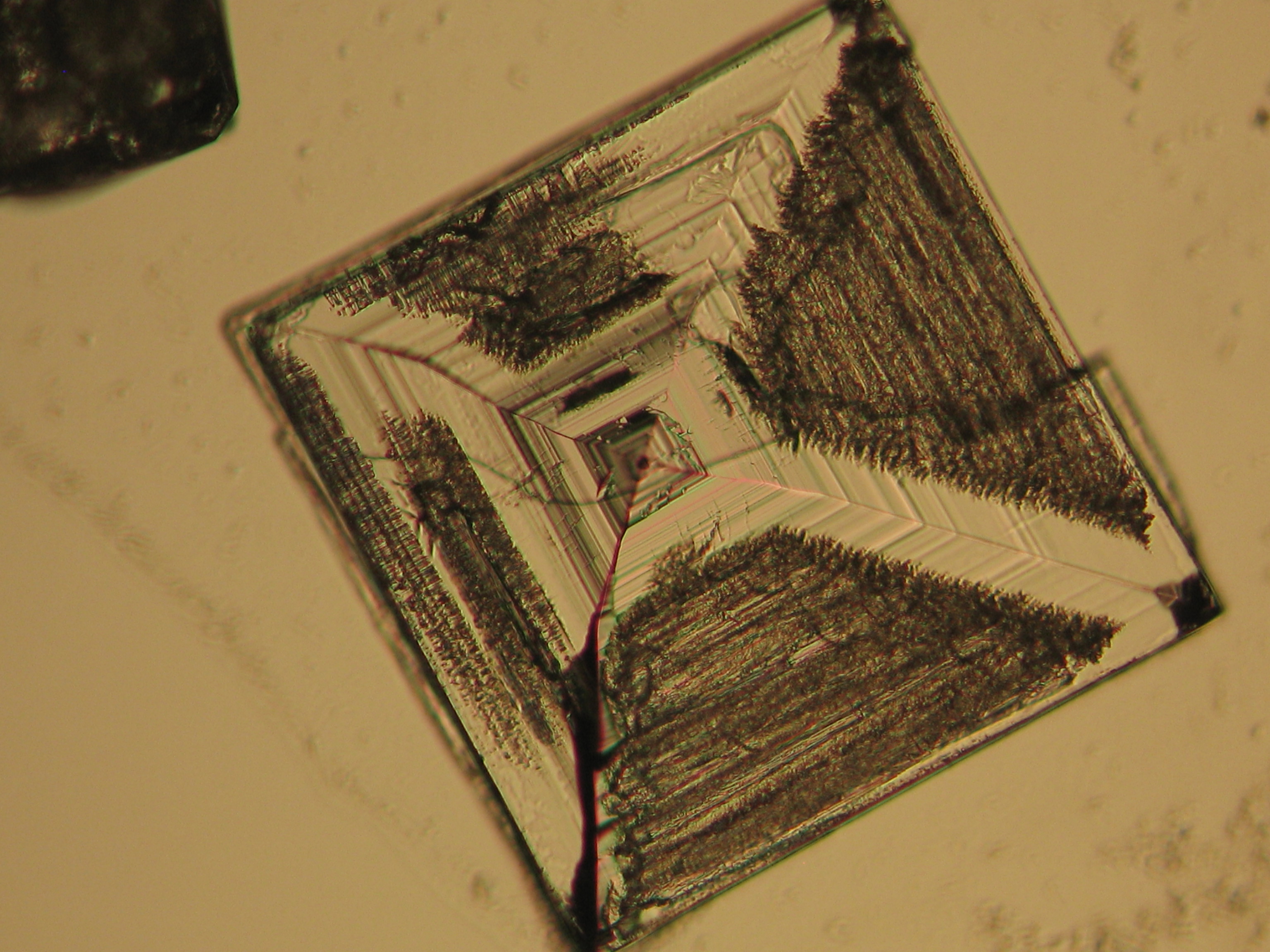
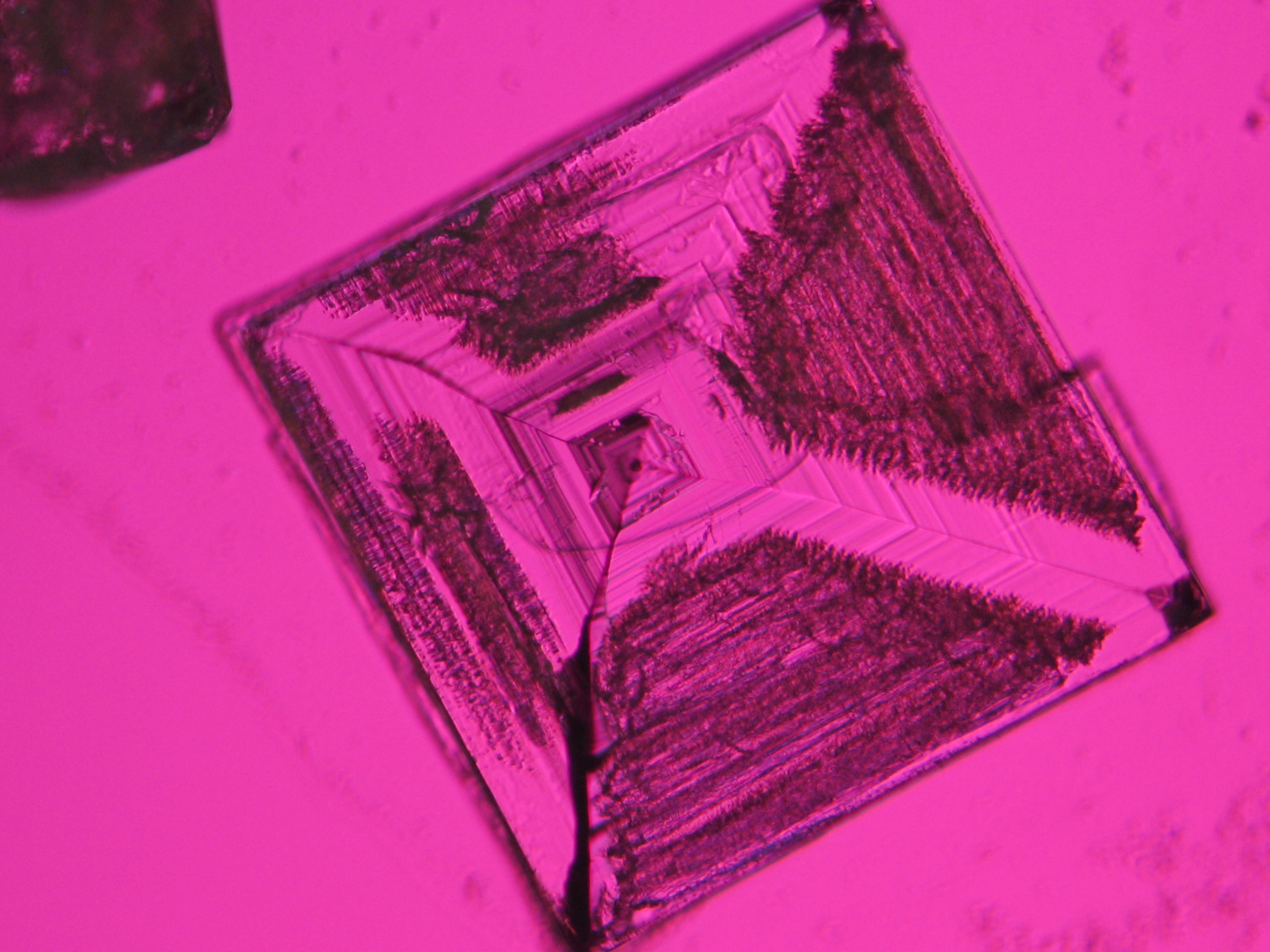
<gallery caption=" | <gallery caption="Precipitated from the evaporation of an aqueous solution of the sample on microscope slides" widths="200px" heights="150px" perrow="3"> | ||
Image:NaCl 27.4.2006-10x (1).JPG|Sodium chloride, precipitate from | Image:NaCl 27.4.2006-10x (1).JPG|Sodium chloride, precipitate from an aqueous solution on microscope slide | ||
Image:NaCl 27.4.2006-10x (2).JPG|Sodium chloride, precipitate from | Image:NaCl 27.4.2006-10x (2).JPG|Sodium chloride, precipitate from an aqueous solution on microscope slide | ||
Image:NaCl 27.4.2006-10x (5).JPG|Sodium chloride, precipitate from | Image:NaCl 27.4.2006-10x (5).JPG|Sodium chloride, precipitate from an aqueous solution on microscope slide | ||
Image:NaCl 27.4.2006-10x (6).JPG|Sodium chloride, precipitate from | Image:NaCl 27.4.2006-10x (6).JPG|Sodium chloride, precipitate from an aqueous solution on microscope slide | ||
Image:NaCl 27.4.2006-10x.JPG| Sodium chloride, precipitate from | Image:NaCl 27.4.2006-10x.JPG| Sodium chloride, precipitate from an aqueous solution on microscope slide | ||
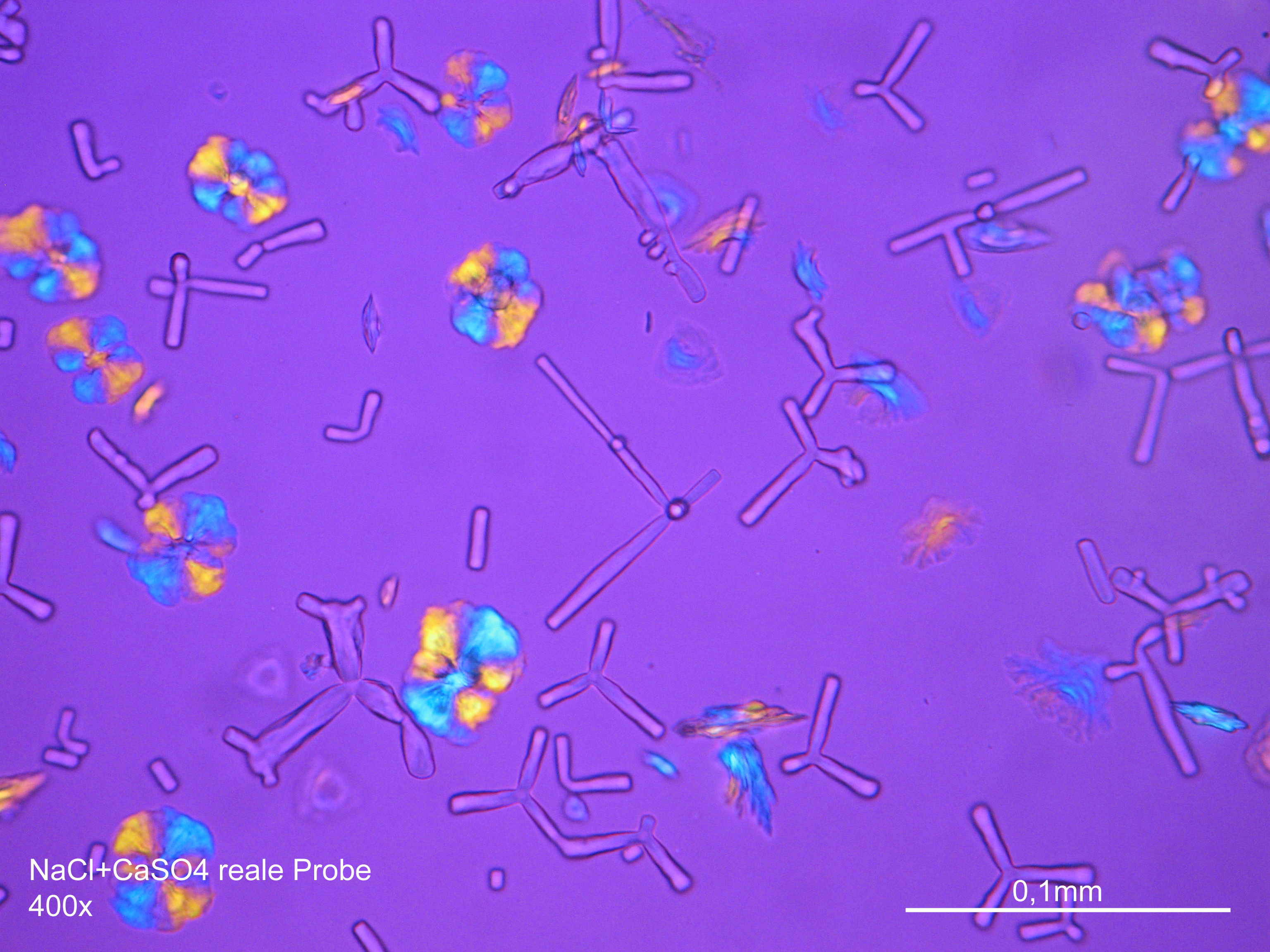
Image:NaCl+CaSO4 reale Probe 01.JPG|Sodium chloride and calcium sulphate in real sample, precipitate from | Image:NaCl+CaSO4 reale Probe 01.JPG|Sodium chloride and calcium sulphate in real sample, precipitate from an aqueous solution on microscopic slide | ||
Image:NaCl+CaSO4 reale Probe 02.JPG|Sodium chloride and calcium sulphate in real sample, precipitate from | Image:NaCl+CaSO4 reale Probe 02.JPG|Sodium chloride and calcium sulphate in real sample, precipitate from an aqueous solution on microscopic slide | ||
</gallery> | </gallery> | ||
=== Scanning Electron Microscope photomicrographs === | |||
<gallery caption="" widths="200px" heights="150px" perrow="2"> | |||
Image:NaCl S.Stefano.jpg|NaCl whisker growing on brick from a church in Venice. The sample had been kept in a film cartridge so it dried out very slowly. A nice cubic crystal can be seen at the tip of the whisker that had been on the surface of the brick as efflorescence when the sample was taken. | |||
</gallery> | |||
== Weblinks == | == Weblinks == | ||
| Line 199: | Line 227: | ||
<!-- System NaCl-H2O was by BRAITSCH (1962) and FRENZEL (1980) --> | <!-- System NaCl-H2O was by BRAITSCH (1962) and FRENZEL (1980) --> | ||
< | <biblist/> | ||
[[Category:Halite]] [[Category: | '''More Literature''' | ||
<!-- | |||
<bibprint filter="title:%NaCl%"/> | |||
--> | |||
[[Category:Halite]] [[Category:Schwarz,Hans-Jürgen]][[Category:Mainusch,Nils]] [[Category:R-MSteiger]] [[Category:Chloride]] [[Category:Salt]][[Category:approved]][[Category:List]] | |||
Latest revision as of 10:24, 29 August 2023
Authors: Hans-Jürgen Schwarz, Nils Mainusch
English version by Christa Gerdwilker
back to Chloride
| Halite[1][2][3] [4] | |

| |
| Mineralogical name | Halite |
| Chemical name | Sodium chloride |
| Trivial name | Common Salt, Rock Salt |
| Chemical formula | NaCl |
| Other forms | NaCl•2H2O (Hydrohalite) |
| Crystal system | cubic |
| Crystal structure | |
| Deliquescence humidity 20°C | 75.4% |
| Solubility (g/l) at 20°C | 6.135 mol/kg |
| Density (g/cm³) | 2.163 g/cm3 |
| Molar volume | 27.02 cm3/mol |
| Molar weight | 58.44 g/mol |
| Transparency | transparent to translucent |
| Cleavage | perfect |
| Crystal habit | cubic crystal, granular, massive aggregates |
| Twinning | none |
| Phase transition | |
| Chemical behavior | |
| Comments | water soluble |
| Crystal Optics | |
| Refractive Indices | nD=1.5443 |
| Birefringence | |
| Optical Orientation | isotropic |
| Pleochroism | |
| Dispersion | |
| Used Literature | |
| [Steiger.etal:2014]Title: Weathering and Deterioration Author: Steiger, Michael; Charola A. Elena; Sterflinger, Katja  [Robie.etal:1978]Title: Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar pressure and higher temperatures [Robie.etal:1978]Title: Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar pressure and higher temperaturesAuthor: Robie R.A., Hemingway B.S.; Fisher J.A.  [Dana:1951]Title: Dana's System of Mineralogy [Dana:1951]Title: Dana's System of MineralogyAuthor: Dana J.D. 
| |
Abstract[edit]
Occurrence[edit]
Sodium chloride is obtained through mining or derived from the sea or salt lakes and is commonly used for cooking or as deicing salt for roads.
The sodium chloride content of sea water is around 2.7 M.%.
Origin of the halite found on monuments[edit]
Sodium chloride can enter buildings or monuments when these are in contact with materials containing this salt or even other salts containing either sodium or chloride, that might combine to produce NaCl in or efflorescence on them. Contamination with sodium and chloride ions can also occur through contact with salt laden ground or surface water. A range of cleaning materials (e.g., acidic and alkaline cleaners or combination of them), or previously used restoration materials (e.g., water glass) as well as Portland cement, can introduce sodium and chloride ions into monuments. Further common and important sources are deicing salts and maritime environments where the air and fogs may contain a significant amount of sodium chloride in suspension or dissolved in droplets.
Solubility behavior[edit]
The commonly occurring halite has a solubility of 6.13 mol/kg (20°C) and can be considered as a very soluble and, therefore, easily mobilized salt. Its solubility changes not significantly within a temperature range of 10 -30°C.
| Temperature | 10°C | 20°C | 30°C | 40°C |
| Solubility [mol/kg] | 6.11 | 6.13 | 6.17 | 6.22 |
Hygroscopicity[edit]
Halite has a deliquescence humidity of about 75% RH, therefore it tends to pick up moisture easily in most temperate climates.
| 0°C | 10°C | 20°C | 30°C | 40°C | 50°C |
| 75.9%r.h. | 75.6%r.h. | 75.4%r.h. | 75.2%r.h. | 75.2%r.h. | 74.8%r.h. |
Moisture sorption:
Theoretically 1g NaCl can take up 4.3g of moisture, i.e., water vapor. The moisture sorption during varying relative humidity levels is:
| Relative humidity during storge/salt phase | NaCl |
| 87% RH | 153 |
| 81% RH | 22 |
| 79% RH | 7 |
Hydration behavior[edit]
Under normal environmental conditions only halite will crystallize out of a saturated solution. The hydrated form, dihydrate hydrohalite[4] will only precipitate out at temperatures below 0.15°C.
Microscopy[edit]
Laboratory analysis[edit]
Sodium chloride crystals can be reliably identified on the basis of their morphological features. Individual particles usually form cubic or octahedral shapes and, therefore, clearly display right angles in their crystal construction.
Refractive index: nD = 1.544
Crystal category: cubic
Examination by polarized microscopy:
There are few salts belonging to the cubic crystal system which can be found in masonry, i.e., sodium chloride (halite), potassium chloride (sylvite) and calcium fluoride (flourite). Only the two first salts are highly soluble and therefore they are the ones that can cause damage to the masonry. Because of its isotropic internal structure these salts do not display birefringence.
The refractive index can be measured with the immersion method by using a standard oil with a refractive index of nD =1.518. Halite crystals display the same optical density in every direction so that the speed and orientation of linear polarized light waves are not distorted, therefore, when viewed between crossed polars, the crystals are not visible, i.e., they show "extinction".
Differentiation of halite from similar salts:
The three above mentioned isotropic salts can be easily differentiated.
| Salt phase | Identification features |
| Sylvine KCl | Refractive index below 1,518. |
| Fluorite CaF2 | Refractive index below 1,518, barely water soluble. |
Images of salts and salt damage[edit]
In situ[edit]
Under the polarizing microscope[edit]
- Precipitated from the evaporation of an aqueous solution of the sample on microscope slides
Scanning Electron Microscope photomicrographs[edit]
Weblinks[edit]
- ↑ http://webmineral.com/data/Halite.shtml accessed 28.07.2010
- ↑ http://www.mindat.org/min-1804.html accessed 28.07.2010
- ↑ http://www.mineralienatlas.de/lexikon/index.php/MineralData?mineral=Halit accessed 28.07.2010
- ↑ 4.0 4.1 http://www.mineralienatlas.de/lexikon/index.php/MineralData?mineral=Hydrohalit accessed 28.07.2010
Literature[edit]
| [Dana:1951] | Dana E.S. (eds.) Dana J.D. (1951): Dana's System of Mineralogy, 7, Wiley & Sons |  |
| [Robie.etal:1978] | Robie R.A., Hemingway B.S.; Fisher J.A. (1978): Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar pressure and higher temperatures. In: U.S. Geol. Surv. Bull, 1452 () |  |
| [Steiger] | The entry doesn't exist yet. | |
| [Steiger.etal:2008c] | Steiger, Michael; Kiekbusch, Jana; Nicolai, Andreas (2008): An improved model incorporating Pitzer’s equations for calculation of thermodynamic properties of pore solutions implemented into an efficient program code. In: Construction and Building Materials, 22 (8), 1841-1850, 10.1016/j.conbuildmat.2007.04.020 |  |
| [Steiger.etal:2014] | Steiger, Michael; Charola A. Elena; Sterflinger, Katja (2014): Weathering and Deterioration. In: Siegesmund S.; Snethlage R. (eds.): Stone in Architecture, Springer Verlag Berlin Heidelberg, 223-316, 10.1007/978-3-642-45155-3_4. |  |
| [Vogt.etal:1993] | Vogt, R.; Goretzki, Lothar (1993): Der Einfluss hygroskopischer Salze auf die Gleichgewichtsfeuchte und Trocknung anorganischer Baustoffe, unveröffentlichter Bericht. |  |
| [Winkler:1975] | Winkler, Erhard M. (1975): Stone: Properties, Durability in Man´s Environment, Springer Verlag, Wien |  |
More Literature